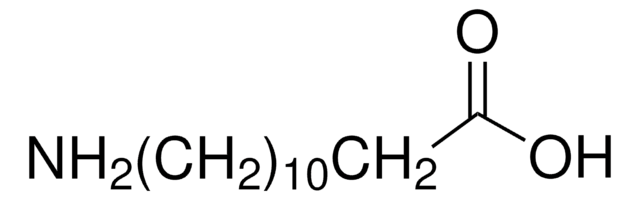198781
12-Hydroxydodecanoic acid
97%
Synonym(s):
12-Hydroxylauric acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HO(CH2)11COOH
CAS Number:
Molecular Weight:
216.32
Beilstein:
1238370
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
85-88 °C (lit.)
functional group
carboxylic acid
hydroxyl
SMILES string
OCCCCCCCCCCCC(O)=O
InChI
1S/C12H24O3/c13-11-9-7-5-3-1-2-4-6-8-10-12(14)15/h13H,1-11H2,(H,14,15)
InChI key
ZDHCZVWCTKTBRY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
12-Hydroxydodecanoic acid was used in the synthesis of high molecular weight poly[(12-hydroxydodecanoate)-co-(12-hydroxystearate)] [poly(12HD-co-12HS)] samples with variable monomer ratios using methyl 12-hydroxystearate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D D Giera et al.
Fundamental and applied toxicology : official journal of the Society of Toxicology, 16(2), 348-355 (1991-02-01)
Assessment of hepatic omega-oxidation of fatty acids by cytochrome P450IV enzymes in toxicology studies can be a means of evaluating test compound effects on peroxisomal proliferation. Routine assay of omega-oxidation, however, requires a simpler method of enzymatic analysis than currently
D E Jensen et al.
The Biochemical journal, 331 ( Pt 2), 659-668 (1998-06-11)
An enzyme isolated from rat liver cytosol (native molecular mass 78. 3 kDa; polypeptide molecular mass 42.5 kDa) is capable of catalysing the NADH/NADPH-dependent degradation of S-nitrosoglutathione (GSNO). The activity utilizes 1 mol of coenzyme per mol of GSNO processed.
P Jezek et al.
FEBS letters, 408(2), 166-170 (1997-05-19)
Fatty acid (FA) uniport via mitochondrial uncoupling protein (UcP) was detected fluorometrically with PBFI, potassium-binding benzofuran phthalate and SPQ, 6-methoxy-N-(3-sulfopropyl)-quinolinium, indicating K+ and H+, respectively. The FA structural patterns required for FA flip-flop, UcP-mediated FA uniport, activation of UcP-mediated H+
H A Dirven et al.
Journal of chromatography, 564(1), 266-271 (1991-03-08)
The formation of omega-hydroxylauric acid from lauric acid is an indicator of the activity of cytochrome P-450 IV family proteins. The two main metabolites of lauric acid, (omega-1)-and omega-hydroxylauric acid, have been completely separated by reversed-phase high-performance liquid chromatography. Measurement
Elena Bailo et al.
Analytical and bioanalytical chemistry, 394(7), 1797-1801 (2009-06-16)
Surface-enhanced Raman scattering was used as a spectroscopic tool to investigate the changes brought upon cytochrome P450BSss after fatty acid binding. Differences in the spectra of substrate-free and substrate-bound enzyme were observed indicating the potential for this method to be
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service