All Photos(2)
About This Item
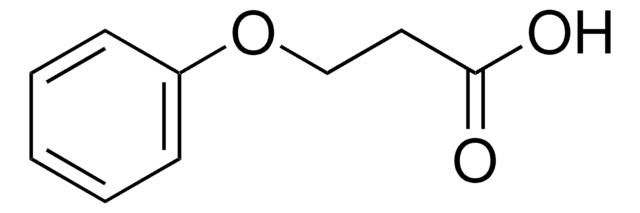
Linear Formula:
C6H5OCH(CH3)CO2H
CAS Number:
Molecular Weight:
166.17
Beilstein:
5734971
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98%
bp
265 °C (lit.)
mp
112-115 °C (lit.)
functional group
carboxylic acid
phenoxy
SMILES string
CC(Oc1ccccc1)C(O)=O
InChI
1S/C9H10O3/c1-7(9(10)11)12-8-5-3-2-4-6-8/h2-7H,1H3,(H,10,11)
InChI key
SXERGJJQSKIUIC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Solvent-induced invesrsion of enantioselectivity during esterification of 2-phenoxypropionic acid catalyzed by Candida cylindracea lipase has been investigated. Derivatives of 2-phenoxypropionic acid are potential herbicides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Solvent-induced inversion of enantiosflectivity in lipase-catalyzed esterification of 2-phenoxypropionic acids.
Ueji S, et al.
Biotechnology Letters, 14(3), 163-168 (1992)
Lipase-catalyzed production of optically active acids via asymmetric hydrolysis of esters.
Cambou B and Klibanov AM.
Applied Biochemistry and Biotechnology, 9(3), 255-260 (1984)
Yan He et al.
Journal of pharmaceutical sciences, 95(1), 97-107 (2005-11-30)
The efficiency of a solubilization technique is determined by the physical-chemical properties of the drug. This study investigates the solubilization on two structurally related anticancer drugs, XK-469 and PPA. XK-469 is much less polar than PPA with an intrinsic solubility
Manuel A P Segurado et al.
The Journal of organic chemistry, 72(14), 5327-5336 (2007-06-15)
Rate constants were measured for the oxidative chlorodehydrogenation of (R,S)-2-phenoxypropanoic acid and nine ortho-, ten para- and five meta-substituted derivatives using (R,S)-1-chloro-3-methyl-2,6-diphenylpiperidin-4-one (NCP) as chlorinating agent. The kinetics was run in 50% (v/v) aqueous acetic acid acidified with perchloric acid
J Haginaka et al.
Enantiomer, 5(1), 37-45 (2000-04-14)
HPLC chiral stationary phases based on human plasma alpha1-acid glycoprotein (AGP) and partially deglycosylated AGP (pd-AGP) were prepared to investigate the effects of sugar moiety of AGP on chiral discrimination of various solutes. Removal of a sugar moiety of AGP
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service