194484
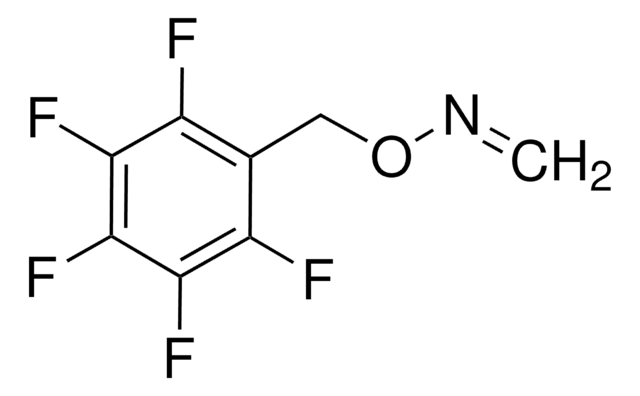
O-(2,3,4,5,6-Pentafluorobenzyl)hydroxylamine hydrochloride
≥98%
Synonym(s):
PFBHA·HCl
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6F5CH2ONH2·HCl
CAS Number:
Molecular Weight:
249.57
Beilstein:
4031190
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98%
form
solid
mp
227 °C (subl.) (lit.)
solubility
water: soluble 50 mg/mL, clear to slightly hazy, colorless
functional group
fluoro
SMILES string
Cl.NOCc1c(F)c(F)c(F)c(F)c1F
InChI
1S/C7H4F5NO.ClH/c8-3-2(1-14-13)4(9)6(11)7(12)5(3)10;/h1,13H2;1H
InChI key
HVMVKNXIMUCYJA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
O-(2,3,4,5,6-Pentafluorobenzyl)hydroxylamine hydrochloride is used:
- In the preparation of oximes of steroids-bearing keto group.
- As a derivatization reagent in the determination of thromboxane B2, prostaglandins, amygdalin, and a variety of aldehydes, ketones, and acids.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Raya Ahmed et al.
Cell reports, 33(11), 108501-108501 (2020-12-17)
A central paradigm in the field of lymphocyte biology asserts that replicatively senescent memory T cells express the carbohydrate epitope CD57. These cells nonetheless accumulate with age and expand numerically in response to persistent antigenic stimulation. Here, we use in vivo deuterium
Vicente Ferreira et al.
Journal of chromatography. A, 1122(1-2), 255-265 (2006-05-20)
This work presents a thorough study of some aspects critical to the quantitative performance of methods for the determination of volatile aldehydes previously derivatized to pentafluorobenzyl hydroxylamine oximes. The conclusions of the study are further applied to the validation of
Chunhui Deng et al.
Rapid communications in mass spectrometry : RCM, 19(5), 647-653 (2005-02-09)
In the current work, a simple, rapid, accurate and inexpensive method was developed for the determination of acetone in human blood. The proposed method is based on derivatization with O-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine hydrochloride (PFBHA), followed by headspace liquid-phase microextraction (HS-LPME) and gas
R S Spaulding et al.
Analytical and bioanalytical chemistry, 372(7-8), 808-816 (2002-05-16)
The employment of O-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine (PFBHA) derivatization along with bis-(trimethylsilyl)trifluoroacetamide (BSTFA) or N, N-( tert-butyldimethylsilyl)trifluoroacetamide (MTBSTFA) derivatization is a popular method for measurement of oxygenated organics in environmental and biological samples. Most notably, the derivatization method enables the measurement of atmospheric
Chunhui Deng et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 810(2), 269-275 (2004-09-24)
Analysis of breath acetone has been used as a diagnostic tool for diabetes. Due to its nature of volatility and activity, it is very difficult to accurately measure the concentration of acetone in human breath by gas chromatography-mass spectrometry (GC-MS).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service