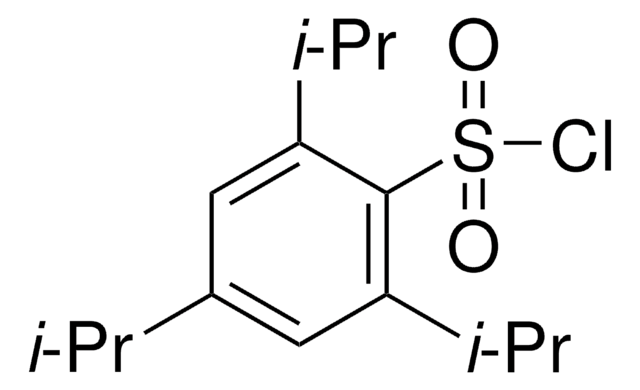
192198
2,4,6-Triisopropylbenzenesulfonyl hydrazide
90%
Synonym(s):
2,4,6-Triisopropylbenzenesulfonohydrazide, TPSH, Trisylhydrazide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[(CH3)2CH]3C6H2SO2NHNH2
CAS Number:
Molecular Weight:
298.44
Beilstein:
2145001
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
90%
form
powder
mp
110-112 °C (dec.) (lit.)
functional group
hydrazine
storage temp.
−20°C
SMILES string
CC(C)c1cc(C(C)C)c(c(c1)C(C)C)S(=O)(=O)NN
InChI
1S/C15H26N2O2S/c1-9(2)12-7-13(10(3)4)15(20(18,19)17-16)14(8-12)11(5)6/h7-11,17H,16H2,1-6H3
InChI key
UGRVYFQFDZRNMQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,4,6-Triisopropylbenzenesulfonyl hydrazide (TPSH) is a best source of diazene. It can be used as a selective reducing agent for the reduction of alkenes and other double bonds via in situ formation of diazene (diimide) in the presence of base. TPSH reduction method is efficiently used in the synthesis of polymers and natural products as it tolerates sensitive groups such as esters, ketones, or organometal complexes. It also undergoes condensation reaction with ketones and aldehydes to produce corresponding hydrazones that can be converted into reactive intermediates such as diazoalkanes, carbenes, carbenium ions, and alkyllithiums.
It can be used as a reagent to synthesize:
It can be used as a reagent to synthesize:
- Nitrogen-containing polycyclic compounds by intramolecular cyclopropanation of N-alkyl indoles/pyrroles.
- Vinyl sulfones by sulfonylation reaction of vinyl bromides.
- Nitrile derivatives from carbonyl compounds via formation of corresponding hydrazones.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
2,4,6-Triisopropylbenzenesulfonylhydrazide
Chamberlin AR, et al.
Encyclopedia of Reagents for Organic Synthesis, Second Edition null
Cobalt (II) Porphyrin-Catalyzed Intramolecular Cyclopropanation of N-Alkyl Indoles/Pyrroles with Alkylcarbene: Efficient Synthesis of Polycyclic N-Heterocycles
Reddy AR, et al.
Angewandte Chemie (International ed. in English), 55(5), 1810-1815 (2016)
2, 4, 6-Tri-isopropylbenzenesulphonyl hydrazide: A convenient source of di-imide
Cusack NJ, et al.
Tetrahedron, 32(17) (1976)
The asymmetric synthesis of cyclopentane derivatives by palladium-catalyzed coupling of prochiral alkylboron compounds.
Cho SY and Shibasaki M.
Tetrahedron Asymmetry, 9(21), 3751-3754 (1998)
Synthesis and x-ray crystal structure of anti-dithia [3.3](2.6) triquinacenophane.
Roberts WP and Shoham G.
Tetrahedron Letters, 22(49), 4895-4898 (1981)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)