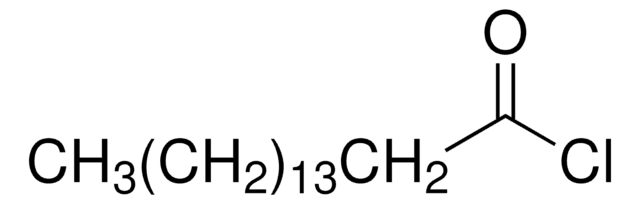171158
Stearoyl chloride
97%
Synonym(s):
Octadecanoyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3(CH2)16COCl
CAS Number:
Molecular Weight:
302.92
Beilstein:
639784
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39050509
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.454 (lit.)
bp
174-178 °C/2 mmHg (lit.)
mp
21-22 °C (lit.)
density
0.897 g/mL at 25 °C (lit.)
functional group
acyl chloride
SMILES string
CCCCCCCCCCCCCCCCCC(Cl)=O
InChI
1S/C18H35ClO/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h2-17H2,1H3
InChI key
WTBAHSZERDXKKZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Stearoyl chloride was used in the synthesis of 4-fluoroceramide. It was also used in the preparation of shimofuridin analogs: 2′-O-(4-O-stearoyl-alpha-L-fucopyranosyl)thymidine and -uridine.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gergana S Nikolova et al.
Beilstein journal of organic chemistry, 4, 12-12 (2008-10-23)
Sphingolipids belong to the most important constituents of the membranes of eukaryotic cells. As intermediates in sphingolipid metabolism, sphingosine and its N-octadecanoyl-derivative, ceramide, exhibit a variety of biological functions. These compounds play a crucial role in many essential biological processes
Jun Ning et al.
Carbohydrate research, 338(1), 55-60 (2002-12-31)
Two shimofuridin analogs: 2'-O-(4-O-stearoyl-alpha-L-fucopyranosyl)thymidine (2) and -uridine (3) have been synthesized using D-arabinose, L-fucose, thymine, uracil, and stearoyl chloride as the starting materials. The synthetic procedures involve the facile preparation of 1-(3,5-di-O-benzyl-beta-D-ribofuranosyl)thymine (9) and -uracil (10) by coupling of 1,2-anhydro-3,5-di-O-benzyl-alpha-D-ribofuranose
A V Kabanov et al.
Biomedical science, 1(1), 63-67 (1990-01-01)
A method is proposed for the inhibition of viral reproduction in cells by means of fatty-acylated antiviral antibodies which, in contrast to the unmodified antibodies, have the ability to enter the cells. The potential of this technique is demonstrated in
A M Ladhoff et al.
Biomedica biochimica acta, 43(7), 963-969 (1984-01-01)
The assembly of electron dense ferritin modified by acylation with steaorylchloride into small and large egg lecithin vesicles is reported. From electron micrographs conclusions are drawn: on the mode of ferritin incorporation in the lipid bilayer: Small liposomes seem to
[Chemical modification of proteins (enzymes) by water-insoluble reagents].
A V Levashov et al.
Doklady Akademii nauk SSSR, 278(1), 246-248 (1984-09-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service