168092
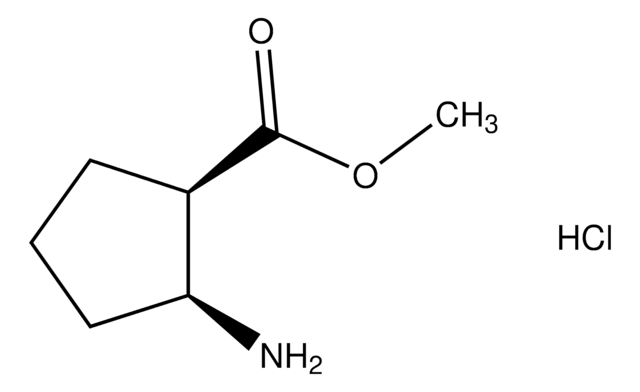
Ethyl 2-oxocyclopentanecarboxylate
95%
Synonym(s):
2-(Ethoxycarbonyl)cyclopentanone, Ethyl cyclopentanone-2-carboxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(O=)C5H7CO2C2H5
CAS Number:
Molecular Weight:
156.18
Beilstein:
387787
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
refractive index
n20/D 1.452 (lit.)
bp
102-104 °C/11 mmHg (lit.)
density
1.054 g/mL at 25 °C (lit.)
functional group
ester
ketone
SMILES string
CCOC(=O)C1CCCC1=O
InChI
1S/C8H12O3/c1-2-11-8(10)6-4-3-5-7(6)9/h6H,2-5H2,1H3
InChI key
JHZPNBKZPAWCJD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Phase-transfer benzylation reaction of ethyl 2-oxocyclopentanecarboxylate with benzyl bromide in microreactor has been investigated. Ethyl 2-oxocyclopentanecarboxylate participates in cobalt (II) Schiff′s base complex catalyzed oxidation of primary and secondary alcohols.
Application
Ethyl 2-oxocyclopentanecarboxylate was used in stereoselective synthesis of (±)-cis,cis-spiro[4.4]nonane-1,6-diol. It was also used in the synthesis of tanikolide.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
170.6 °F - closed cup
Flash Point(C)
77 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A new synthesis of tanikolide.
Zhai H, et al.
Tetrahedron Letters, 44(14), 2893-2894 (2003)
Masaharu Ueno et al.
Chemical communications (Cambridge, England), (8)(8), 936-937 (2003-05-15)
Phase-transfer alkylation in a microreactor proceeds smoothly, and the reaction has been found to be more efficient than that in a round-bottomed flask with vigorous stirring; we have observed by an optical microscope study that an interfacial area provided by
Cobalt (II) Schiff's base complex catalysed oxidation of alcohols with dioxygen in the presence of ethyl 2-oxocyclopentanecarboxylate.
Punniyamurthy T and Iqbal J.
Tetrahedron Letters, 35(23), 4007-4010 (1994)
An improved synthesis and resolution of (?)-< i> cis, cis</i>-spiro [4.4] nonane-1, 6-diol.
Nieman JA, et al.
Tetrahedron Asymmetry, 4(9), 1973-1976 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service