155713
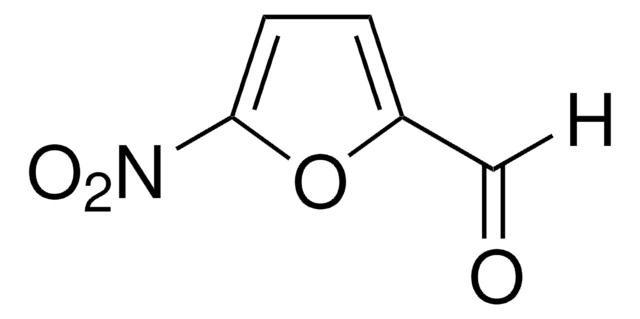
5-Nitro-2-furoic acid
98%
Synonym(s):
5-Nitrofuran-2-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H3NO5
CAS Number:
Molecular Weight:
157.08
Beilstein:
139373
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
185-189 °C (lit.)
functional group
carboxylic acid
nitro
SMILES string
OC(=O)c1ccc(o1)[N+]([O-])=O
InChI
1S/C5H3NO5/c7-5(8)3-1-2-4(11-3)6(9)10/h1-2H,(H,7,8)
InChI key
IODMEDPPCXSFLD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The FT-IR and FT-Raman spectra of 5-Nitro-2-furoic acid has been studied.
Application
5-Nitro-2-furoic acid was used in the preparation of [1,2,4]triazolo[3,4-b][1,3,4]thiadiazines and [1,2,4]triazolo[3,4-b][1,3,4] thiadiazoles.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
V Balachandran et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 113, 268-280 (2013-06-06)
In this work, FT-IR and FT-Raman spectra are recorded on the solid phase of 5-nitro-2-furoic acid (abbreviated as NFA) in the regions 4000-400 cm(-1) and 3500-100 cm(-1) respectively. The geometrical parameters, vibrational assignments, HOMO-LUMO energies and NBO calculations are obtained
R W Busker et al.
Toxicology, 51(2-3), 255-266 (1988-10-01)
The antibacterial drug nitrofurantoin (NFT) is notorious for causing hemolytic anemia, which may be related to the methemoglobinemia, another side-effect of NFT. As NFT is photolabile, and nitrite, well known as a MetHb generator, is an important photoproduct of NFT
Sahar M I Badr et al.
Bioorganic & medicinal chemistry, 19(15), 4506-4512 (2011-07-05)
New series of fused 1,2,4-triazoles such as, 6-(aryl)-3-(5-nitrofuran-2-yl)-5,6-dihydro-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazoles 4-8, 6-(alkyl/aryl amino)-3-(5-nitrofuran-2-yl)-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazoles 9-13 and 6-(4-substituted phenyl)-3-(5-nitrofuran-2-yl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines 14-18 have been synthesized via the reaction of 4-amino-5-(5-nitrofuran-2-yl)-4H-1,2,4-triazole-3-thiol 3 with various reagents such as hetero aromatic aldehydes, alkyl/aryl isothiocyanates and 4-substituted phenacyl bromides, respectively.
Pak Hin Chow et al.
Molecular pharmacology, 98(1), 38-48 (2020-05-22)
Aquaporin-1 (AQP1) dual water and ion channels enhance migration and invasion when upregulated in leading edges of certain classes of cancer cells. Work here identifies structurally related furan compounds as novel inhibitors of AQP1 ion channels. 5-Hydroxymethyl-2-furfural (5HMF), a component
Dharmarajan Sriram et al.
Bioorganic & medicinal chemistry letters, 20(15), 4313-4316 (2010-07-10)
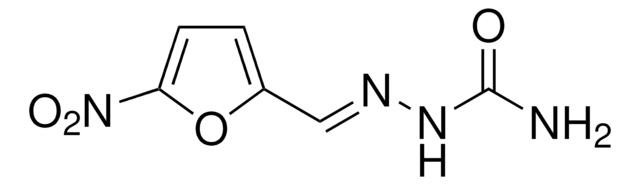
Various 5-nitro-2-furoic acid hydrazones were synthesized and evaluated for in vitro activities against log and starved phase culture of two mycobacterial species and Mycobacterium tuberculosis (MTB) isocitrate lyase (ICL) enzyme inhibition studies. Among twenty one compounds, 5-nitro-N'-[(5-nitro-2-furyl)methylidene]-2-furohydrazide (4o) was found
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service