14625
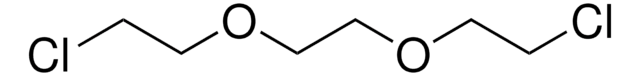
Bis[2-(2-chloroethoxy)ethyl] ether
≥99.0% (T)
Synonym(s):
1,11-Dichloro-3,6,9-trioxaundecane, Tetraethylene glycol dichloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O(CH2CH2OCH2CH2Cl)2
CAS Number:
Molecular Weight:
231.12
Beilstein:
1071710
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0% (T)
refractive index
n20/D 1.464
density
1.18 g/mL at 20 °C (lit.)
functional group
chloro
ether
SMILES string
ClCCOCCOCCOCCCl
InChI
1S/C8H16Cl2O3/c9-1-3-11-5-7-13-8-6-12-4-2-10/h1-8H2
InChI key
ZCFRYTWBXNQVOW-UHFFFAOYSA-N
Application
Bis[2-(2-chloroethoxy)ethyl] ether was used in preparation of series of para-(1,1,3,3-tetramethylbutyl)-phenoxypoly(ethoxy)ethanols. It was used in the synthesis of 1-(4-pyridiniumaldoxime)-2-(((2-chloroethoxy)-2-ethoxy)ethoxy)ethane.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Dermal
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anna Hawrył et al.
Molecules (Basel, Switzerland), 25(18) (2020-09-24)
The aim of this study was to evaluate the ability of multivariate techniques to predict antioxidant and cytotoxic activity of the selected lichens from the chromatographic data. A simple and reproducible HPLC-DAD technique has been used to obtain the chromatographic
The preparation of a series of molecularly homogeneous para-t-octylphenoxypoly (ethoxy) ethanols.
Mansfield RC and Locke JE.
Journal of the American Oil Chemists' Society, 41(4), 267-272 (1964)
M C de Koning et al.
Bioorganic & medicinal chemistry, 19(1), 588-594 (2010-11-30)
A conceptually novel approach to the design of reactivators of nerve agent-inhibited acetylcholinesterase (AChE) is presented. The concept comprises the linkage of a peripheral site ligand via a spacer to a reactivating moiety with the eventual goal to develop non-ionic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![2-[2-(2-Chloroethoxy)ethoxy]ethanol 96%](/deepweb/assets/sigmaaldrich/product/structures/902/295/ff6d7bb1-a7e0-4582-86e1-819084626e67/640/ff6d7bb1-a7e0-4582-86e1-819084626e67.png)



![2-[2-(2-Aminoethoxy)ethoxy]ethanol ≥96.0% (GC)](/deepweb/assets/sigmaaldrich/product/structures/237/185/b94eadd2-5a2c-4a5b-a13d-3d3905df0dbc/640/b94eadd2-5a2c-4a5b-a13d-3d3905df0dbc.png)

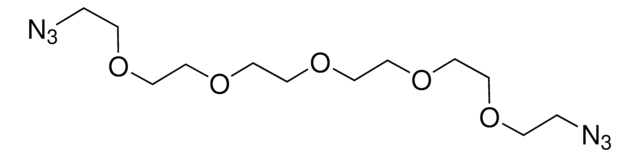
![2-[2-(2-Azidoethoxy)ethoxy]ethanol solution ~0.5 M in tert-butyl methyl ether](/deepweb/assets/sigmaaldrich/product/structures/374/007/eea7ca74-41e4-4aac-af71-c93c37ec0a5a/640/eea7ca74-41e4-4aac-af71-c93c37ec0a5a.png)

