142875
2,6-Pyridinedicarbonyl dichloride
97%
Synonym(s):
2,6-Pyridinedicarbonyl chloride, Pyridine-2,6-dicarboxylic acid chloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H3Cl2NO2
CAS Number:
Molecular Weight:
204.01
Beilstein:
131556
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
284 °C (lit.)
mp
56-58 °C (lit.)
functional group
acyl chloride
SMILES string
ClC(=O)c1cccc(n1)C(Cl)=O
InChI
1S/C7H3Cl2NO2/c8-6(11)4-2-1-3-5(10-4)7(9)12/h1-3H
InChI key
GWHOGODUVLQCEB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,6-Pyridinedicarbonyl dichloride can be used as a starting material to synthesize:
- Pyridine-based polyamido-polyester optically active macrocycles by reacting with chiral diamine dihydrobromides.
- Pyridine-bridged 2,6-bis-carboxamide Schiff′s bases by treating with L-alanine or 2-methyl-alanine methyl esters.
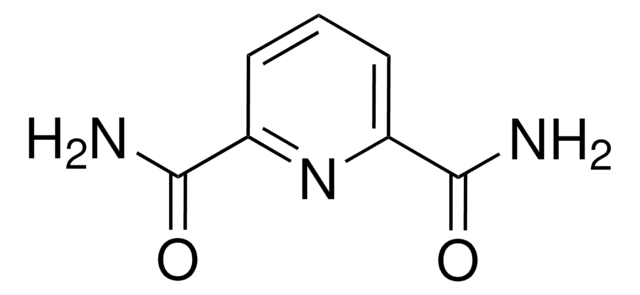
- N,N′-bis(1-pyrenylmethyl)pyridine-2,6-dicarboxamide by reacting with 1-pyrenemethylamine hydrochloride in the presence of a base.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Skin Sens. 1A
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of some new pyridine-2, 6-carboxamide-derived Schiff bases as potential antimicrobial agents
Al-Omar MA and Amr Abd El-Galil E
Molecules (Basel), 15(7), 4711-4721 (2010)
Mohamed A Al-Omar et al.
Molecules (Basel, Switzerland), 15(7), 4711-4721 (2010-07-27)
A series of pyridine-bridged 2,6-bis-carboxamide Schiff's bases has been prepared starting from 2,6-pyridinedicarbonyl dichloride (1) and L-alanine or 2-methyl-alanine methyl ester.The coupling of acid chloride 1 with L-alanine methyl ester hydrochloride -or 2-methylalanine methyl ester hydrochloride gave the corresponding 2,6-bis-carboxamide
H Zhao et al.
The Journal of organic chemistry, 65(10), 2933-2938 (2000-05-18)
Five new chiral macrocycles, 3a-e, have been prepared by the acylation cyclization of chiral diamine dihydrobromide intermediates 2a-c with 2,6-pyridinedicarbonyl dichloride in highly diluted solution at room temperature. The chiral diesters 1a-c needed for the preparation of the macrocycles were
Synthesis and characterization of pyridine-based polyamido-polyester optically active macrocycles and enantiomeric recognition for D-and L-amino acid methyl ester hydrochloride
Zhao H and Hua W
The Journal of Organic Chemistry, 65(10), 2933-2938 (2000)
Recognition of sequence-information in synthetic copolymer chains by a conformationally-constrained tweezer molecule
Colquhoun HM, et al.
Faraday Discussions, 143(7), 205-220 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service