139300
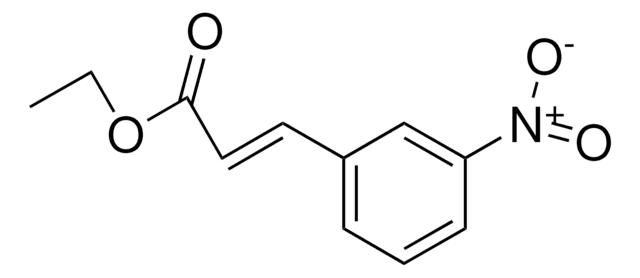
Ethyl 4-nitrocinnamate, predominantly trans
99%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O2NC6H4CH=CHCO2C2H5
CAS Number:
Molecular Weight:
221.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
powder
mp
138-140 °C (lit.)
functional group
ester
nitro
SMILES string
CCOC(=O)\C=C\c1ccc(cc1)[N+]([O-])=O
InChI
1S/C11H11NO4/c1-2-16-11(13)8-5-9-3-6-10(7-4-9)12(14)15/h3-8H,2H2,1H3/b8-5+
InChI key
PFBQVGXIMLXCQB-VMPITWQZSA-N
Related Categories
Application
Ethyl 4-nitrocinnamate was used to study the kinetics of reduction of ethyl t-cinnamate and its substituted derivatives by samarium diiodide in the presence of hexamethylphosphoramide and t-butanol.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Reduction of Ethyl t-cinnamates by Samarium Diiodide
Lin T-Y, et al.
J. Chin. Chem. Soc., 48(5) (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service