All Photos(1)
About This Item
Linear Formula:
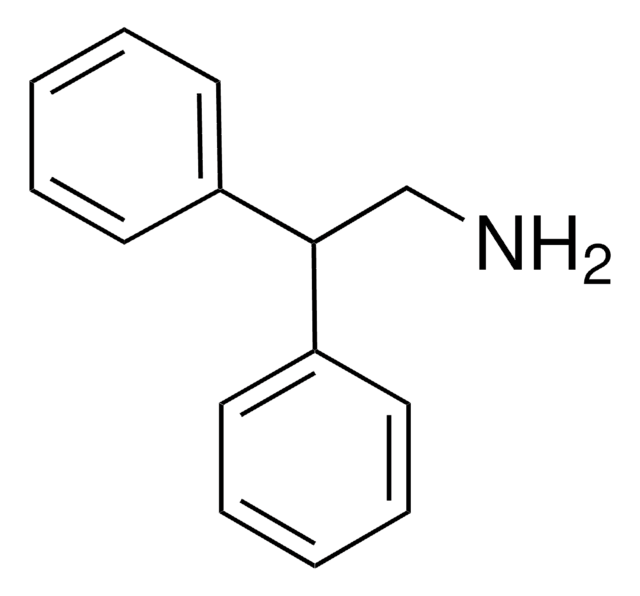
(C6H5)2CHCH2CH2NH2
CAS Number:
Molecular Weight:
211.30
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.583 (lit.)
mp
29-31 °C (lit.)
functional group
amine
phenyl
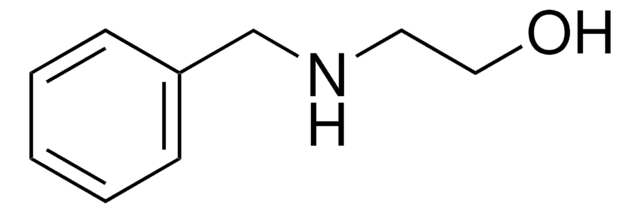
SMILES string
NCCC(c1ccccc1)c2ccccc2
InChI
1S/C15H17N/c16-12-11-15(13-7-3-1-4-8-13)14-9-5-2-6-10-14/h1-10,15H,11-12,16H2
InChI key
KISZTEOELCMZPY-UHFFFAOYSA-N
Application
3,3-Diphenylpropylamine was used as internal standard for simultaneous determination of D- and L-modafinil in human plasma using stereospecific high-performance liquid chromatographic method. It was used as starting reagent in the synthesis of 3,3-diphenylpropylisocyanate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
B Elsenhans et al.
Biochimica et biophysica acta, 813(1), 25-32 (1985-02-28)
Cationic, lipid-soluble organic compounds may interfere with cation-mediated membrane transport processes. Thus, small intestinal absorption may be influenced by lipophilic organic cations. Therefore a series of arylalkylamines was studied in the concentration range from 0.5 to 20 mmol/l for their
A L Mueller et al.
Annals of the New York Academy of Sciences, 890, 450-457 (2000-02-11)
NPS 1506 is a moderate affinity, uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist. NPS 1506 is neuroprotective in rodent models of ischemic stroke, hemorrhagic stroke, and head trauma, with a 2-hr window of opportunity. Neuroprotectant doses of NPS 1506 ranged from approximately
Shiv K Sharma et al.
Journal of medicinal chemistry, 53(14), 5197-5212 (2010-06-24)
The recently discovered enzyme lysine-specific demethylase 1 (LSD1) plays an important role in the epigenetic control of gene expression, and aberrant gene silencing secondary to LSD1 overexpression is thought to contribute to the development of cancer. We recently reported a
S H Gorman
Journal of chromatography. B, Biomedical sciences and applications, 730(1), 1-7 (1999-08-07)
Modafinil, DL-2-[(diphenylmethyl)sulfinyl]acetamide (Provigil), which is chiral at its sulfur atom, is a novel wake-promoting agent currently being developed as the racemate in the United States by Cephalon, Inc. In order to characterize the pharmacokinetic properties of each enantiomer, a stereospecific
T F Holzman et al.
Biochemistry, 20(19), 5524-5528 (1981-09-15)
We have found a new class of inhibitors of the bacterial bioluminescence reaction, the N,N-diphenylalkylamines and acids. We have studied the action of one of these compounds 2,2-diphenylpropylamine. The amine was competitive with the long-chain aliphatic aldehyde substrate (Ki congruent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service