117714
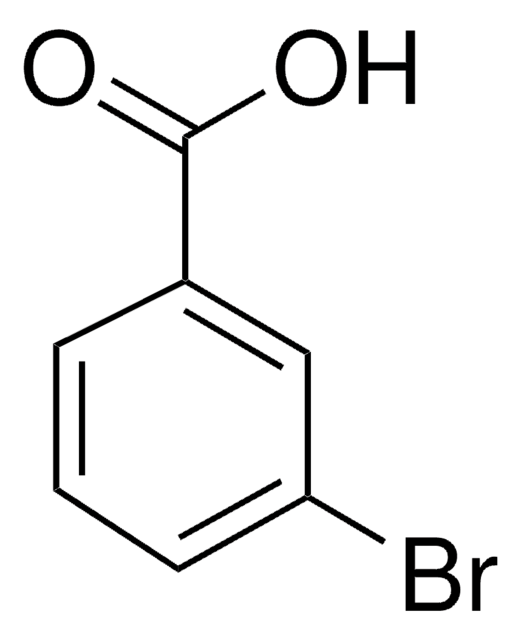
3-Methoxybenzoic acid
ReagentPlus®, 99%
Synonym(s):
m-Anisic acid, m-Methylsalicylic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3OC6H4CO2H
CAS Number:
Molecular Weight:
152.15
Beilstein:
508838
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
bp
170-172 °C/10 mmHg (lit.)
mp
105-107 °C (lit.)
solubility
95% ethanol: soluble 50 mg/mL, clear, colorless to faintly yellow
functional group
carboxylic acid
SMILES string
COc1cccc(c1)C(O)=O
InChI
1S/C8H8O3/c1-11-7-4-2-3-6(5-7)8(9)10/h2-5H,1H3,(H,9,10)
InChI key
XHQZJYCNDZAGLW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Methoxybenzoic acid is an important intermediate in the synthesis of natural products.
Application
3-Methoxybenzoic acid was used in the synthesis and characterization of 3-methoxybenzoates of europium (III) and gadolinium (III). It was used in conversion of aromatic carboxylic acids into methyl esters and reduction to the corresponding primary alcohols using a sodium borohydride-THF-methanol system.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, characterization and thermal behaviour of solid-state compounds of europium (III) and gadolinium (III) 3-methoxybenzoate.
Dametto PR, et al.
Journal of Thermal Analysis and Calorimetry, 97(2), 765-768 (2009)
Sodium borohydride reduction of aromatic carboxylic acids via methyl esters.
Saeed A and Ashraf Z.
Journal of Chemical Sciences (Bangalore), 118(5), 419-423 (2006)
Thi-Huu Nguyen et al.
Organic letters, 7(12), 2445-2448 (2005-06-04)
[reaction: see text] If employed in THF at 0 degrees C, LTMP metalates meta-anisic acid at the doubly activated position. In contrast, n-BuLi/t-BuOK deprotonates position C-4 preferentially at low temperature. Functionalization at C-6 requires protection of the C-2 site beforehand.
A S Waldman et al.
Nucleic acids research, 19(21), 5943-5947 (1991-11-11)
We determined the effect of 3-methoxybenzamide (3-MB), a competitive inhibitor of poly(ADP-ribose) polymerase (E.C. 2.4.2.30), on intrachromosomal homologous recombination in mouse Ltk- cells. We used a cell line that contained in its genome two defective Herpes thymidine kinase (tk) genes
D H Lee et al.
Xenobiotica; the fate of foreign compounds in biological systems, 29(9), 909-916 (1999-11-05)
1. 2-(Allylthio)pyrazine (2-AP) has been demonstrated to protect the liver against toxicants by inhibiting CYP2E1 activity. Since 2-mercaptopyrazine (2-MP) is presumed to be a metabolite of 2-AP, the experiments were performed to determine whether rat liver microsomal and/or cytosolic preparations
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








![Benzo[a]fluorenone BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/881/090/eae85258-97ed-4de7-90c1-c0e0e495552e/640/eae85258-97ed-4de7-90c1-c0e0e495552e.png)

