L6785
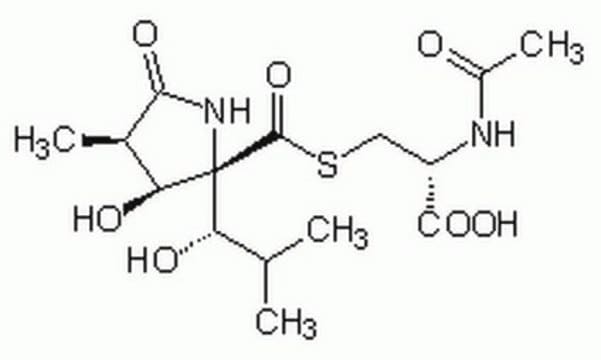
Lactacystin
≥90% (HPLC), powder, proteosome inhibitor
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H24N2O7S
CAS Number:
Molecular Weight:
376.43
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Product Name
Lactacystin, ≥90% (HPLC)
Quality Level
Assay
≥90% (HPLC)
form
powder
potency
4 nM Ki (proteasome inhibitor)
solubility
water: 10 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
CC(C)[C@H](O)[C@]1(NC(=O)[C@H](C)[C@@H]1O)C(=O)SC[C@H](NC(C)=O)C(O)=O
InChI
1S/C15H24N2O7S/c1-6(2)10(19)15(11(20)7(3)12(21)17-15)14(24)25-5-9(13(22)23)16-8(4)18/h6-7,9-11,19-20H,5H2,1-4H3,(H,16,18)(H,17,21)(H,22,23)/t7-,9+,10+,11+,15-/m1/s1
InChI key
DAQAKHDKYAWHCG-RWTHQLGUSA-N
General description
Lactacystin is an antibiotic and a metabolite of Streptomyces spp.
Application
Lactacystin has been used:
- as a proteasome inhibitor to inhibit protein degradation
- to inhibit proteasomal activity of cells for live cell imaging
- to block proteasomal proteolysis in human monocyte-derived dendritic cells (MoDCs) for 24 h
- to provide unilateral injection to animals to induce nigrostriatal lesions
Biochem/physiol Actions
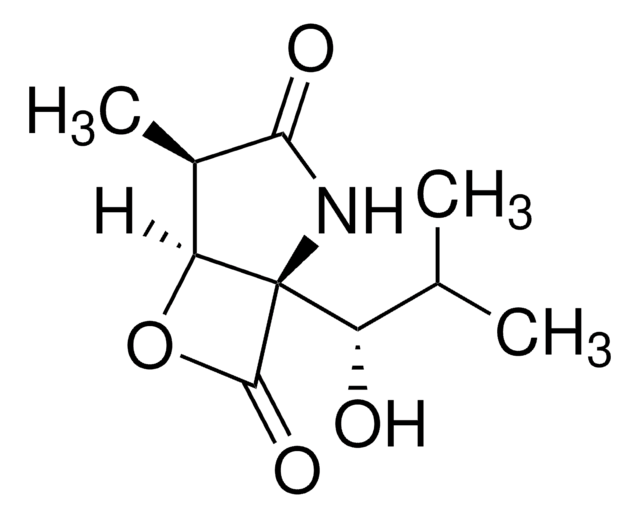
Lactacystin can block the development of cell cycle and stimulate differentiation in a murine neuroblastoma cell line. It can serve as a precursor for clasto-lactacystin β-lactone. Cell-permeable and irreversible proteasome inhibitor (Ki = 4nM). Inhibits NF-kB activation (IC50 = 10mM). Induces neurite outgrowth in neuro2A mouse neuroblastoma cells.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The development and pharmacology of proteasome inhibitors for the management and treatment of cancer
Ruggeri B, et al.
Advances in Pharmacology, 57(13), 91-135 (2009)
Mechanistic Studies on the Inactivation of the Proteasome by Lactacystin A CENTRAL ROLE FOR clasto-LACTACYSTIN beta-LACTONE
Dick L R, et al.
The Journal of Biological Chemistry, 271(13), 7273-7276 (1996)
Evolution of extra-nigral damage predicts behavioural deficits in a rat proteasome inhibitor model of Parkinson's disease
Vernon A C, et al.
PLoS ONE, 6(2), e17269-e17269 (2011)
Differential regulation of MHC II and invariant chain expression during maturation of monocyte-derived dendritic cells
Landsverk O J B, et al.
Journal of Leukocyte Biology, 91(5), 729-737 (2012)
Dallas S Shi et al.
The Journal of clinical investigation, 124(9), 3757-3766 (2014-07-26)
The proteasome inhibiter bortezomib has been successfully used to treat patients with relapsed multiple myeloma; however, many of these patients become thrombocytopenic, and it is not clear how the proteasome influences platelet production. Here we determined that pharmacologic inhibition of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service