SCR544
Human STEMCCA Constitutive Polycistronic (OKSM) Lentivirus Reprogramming Kit
The Human STEMCCA Constitutive Polycistronic Lentivirus Kit contains high titer polycistronic lentivirus & Polybrene transfection reagent that have been validated for the generation of human iPS cells from human foreskin fibroblasts.
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352207
eCl@ss:
32161000
NACRES:
NA.71
Recommended Products
Quality Level
manufacturer/tradename
STEMCCA
technique(s)
cell culture | stem cell: suitable
input
sample type induced pluripotent stem cell(s)
shipped in
dry ice
General description
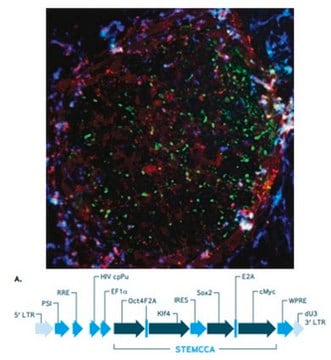
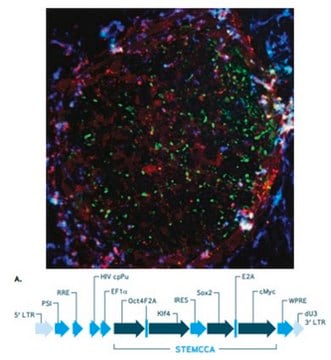
The Human STEMCCA Constitutive Polycistronic (OKSM) Lentivirus Kit contains high titer polycistronic (OKSM) lentivirus and Polybrene transfection reagent that have been validated for the generation of human induced pluripotent stem cells from human foreskin fibroblasts (HFFs). The STEMCCA vector is comprised of the transcription factors human Oct-4, Klf4, SOX-2, and c-Myc (OKSM), separated by the self-cleaving 2A peptide and IRES sequences (Sommer CA, 2009). The use of a single polycistronic lentiviral vector significantly improves reprogramming efficiencies and reduces the number of viral integrations.
Components
1. Human STEMCCA Constitutive (OKSM) Lentivirus: (Part number SCR544-1) Two (2) vials of EF1α-hSTEMCCA (OKSM) Lentivirus (Part number CS204502). Each vial contains 15 µL of high titer lentivirus.
2. Polybrene Transfection Reagent: (Part number TR-1003-50UL) One (1) vial containing 50 µL of 10 mg/mL stock.
2. Polybrene Transfection Reagent: (Part number TR-1003-50UL) One (1) vial containing 50 µL of 10 mg/mL stock.
Quality
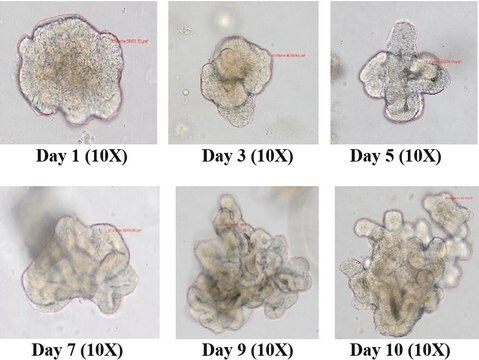
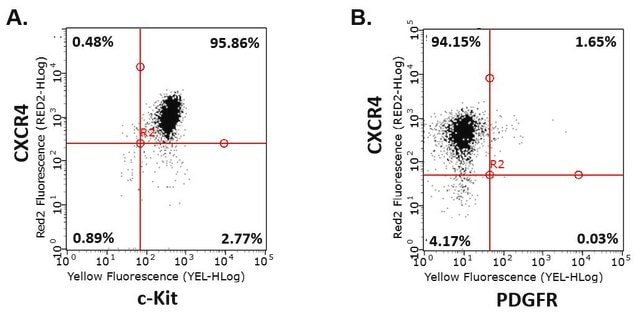
Tested to confirm the generation of iPS cells from p6 human foreskin fibroblasts. Other cell types have not been tested and thus similar results can not be guaranteed.
Storage Class Code
12 - Non Combustible Liquids
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yang Yu et al.
Scientific reports, 5, 10114-10114 (2015-05-13)
Human pluripotent stem cells, including cloned embryonic and induced pluripotent stem cells, offer a limitless cellular source for regenerative medicine. However, their derivation efficiency is limited, and a large proportion of cells are arrested during reprogramming. In the current study
Frank Griscelli et al.
Oncotarget, 10(28), 2693-2708 (2019-05-21)
Recent development of cell reprogramming technologies brought a major hope for future cell therapy applications by the use of these cells or their derivatives. For this purpose, one of the major requirements is the absence of genomic alterations generating a
Jun-Quan Li et al.
Molecular medicine reports, 9(3), 837-842 (2014-01-09)
Myocardial infarction (MI) is an increasing medical problem; however, its pathogenesis has yet to be elucidated and more effective treatment strategies are required. Induced pluripotent stem cells (iPSCs) were recently successfully generated using human somatic cells transfected with four transcription
Martin Madill et al.
Molecular brain, 10(1), 22-22 (2017-06-15)
Amyotrophic lateral sclerosis, a devastating neurodegenerative disease, is characterized by the progressive loss of motor neurons and the accumulation of misfolded protein aggregates. The latter suggests impaired proteostasis may be a key factor in disease pathogenesis, though the underlying mechanisms
Areechun Sotthibundhu et al.
Stem cell research & therapy, 7(1), 166-166 (2016-11-17)
Cellular reprogramming is a stressful process, which requires cells to engulf somatic features and produce and maintain stemness machineries. Autophagy is a process to degrade unwanted proteins and is required for the derivation of induced pluripotent stem cells (iPSCs). However
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service