All Photos(4)
About This Item
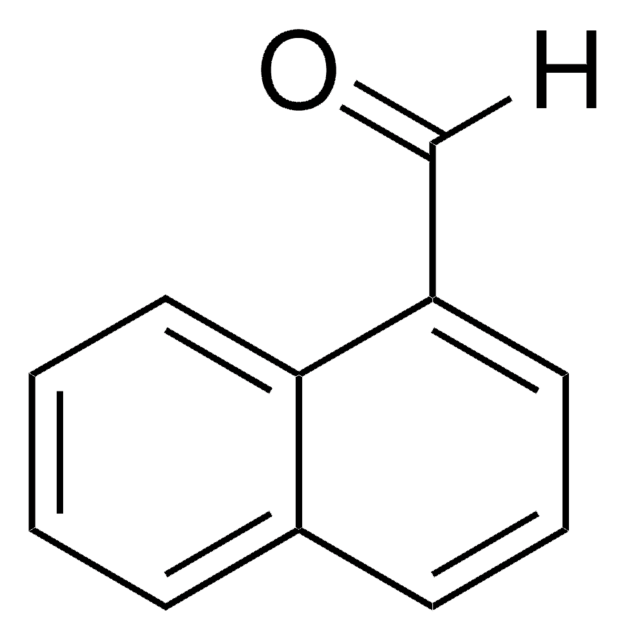
Linear Formula:
C10H7CHO
CAS Number:
Molecular Weight:
156.18
Beilstein:
507750
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
crystals
mp
58-61 °C (lit.)
storage temp.
−20°C
SMILES string
[H]C(=O)c1ccc2ccccc2c1
InChI
1S/C11H8O/c12-8-9-5-6-10-3-1-2-4-11(10)7-9/h1-8H
InChI key
PJKVFARRVXDXAD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Naphthaldehyde can be used as a reactant:
- In proline catalyzed aldol reaction.
- In asymmetric three-component Mannich reaction.
- For the synthesis of Hantzsch 1,4-dihydropyridines.13}
- Asymmetric benzoin condensation reaction.
- For the synthesis of pyrazolo[1,2−b]phthalazinediones.
- For the synthesis C60 by flash vacuum pyrolysis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Proline-catalyzed direct asymmetric aldol reactions.
List B, et al.
Journal of the American Chemical Society, 122(10), 2395-2396 (2000)
Amelioration of H4 [W12SiO40] by nanomagnetic heterogenization: For the synthesis of 1H-pyrazolo [1, 2-b] phthalazinedione derivatives.
Arora P and Rajput JK
Applied Organometallic Chemistry, 32(2), e4001-e4001 (2018)
The direct catalytic asymmetric three-component Mannich reaction.
List B
Journal of the American Chemical Society, 122(38), 9336-9337 (2000)
Zhipeng Zhang et al.
Nature communications, 7, 12478-12478 (2016-08-18)
Due to the high versatility of chiral cyanohydrins, the catalytic asymmetric cyanation reaction of carbonyl compounds has attracted widespread interest. However, efficient protocols that function at a preparative scale with low catalyst loading are still rare. Here, asymmetric counteranion-directed Lewis
Dong-Sheng Lee et al.
Chirality, 28(1), 65-71 (2015-10-22)
Chiral O,N,O-tridentate phenol ligands bearing a camphor backbone were found to be effective chiral catalysts for the enantioselective addition of diethylzinc to aromatic aldehydes, resulting in high enantioselectivities (80-95% ee) at room temperature.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service