514004
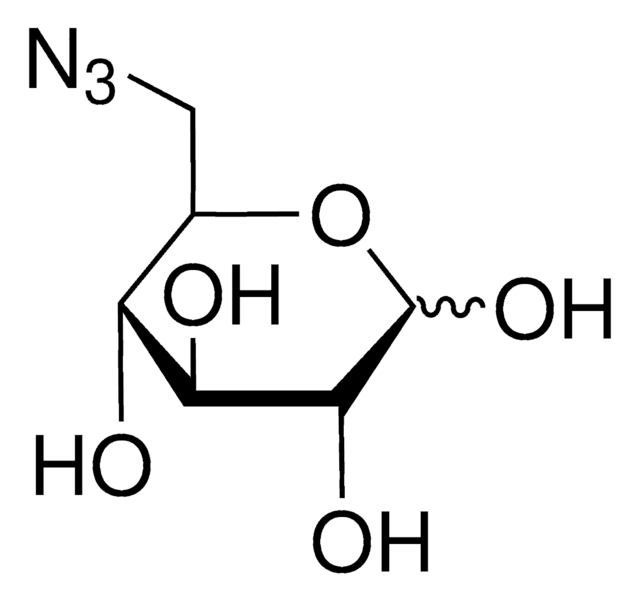
1-Azido-1-deoxy-β-D-glucopyranoside
Synonym(s):
Β-D-Glycopyranosyl azide, 1-Azido-1-deoxy-beta-D-glucose
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H11N3O5
CAS Number:
Molecular Weight:
205.17
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
Quality Level
reaction suitability
reaction type: click chemistry
mp
60-65 °C (lit.)
SMILES string
OC[C@H]1O[C@@H](N=[N+]=[N-])[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C6H11N3O5/c7-9-8-6-5(13)4(12)3(11)2(1-10)14-6/h2-6,10-13H,1H2/t2-,3-,4+,5-,6-/m1/s1
InChI key
KSRDTSABQYNYMP-VFUOTHLCSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-Azido-1-deoxy-β-D-glucopyranoside can be used as an organic building block to prepare:
- 1,2,3-triazole-containing (1-azido-1-deoxy-β-D-glucopyranoside) complex by reacting with propagylglycine and sodium ascorbate via copper-catalyzed click chemistry approach.
- D-glucose-mercaptoacetyl triglycine (MAG3) derivative applicable as a potent tumor diagnosis agent.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S K Sinha et al.
Carbohydrate research, 81(2), 239-247 (1980-03-15)
Syntheses are reported of 4-deoxy-D-xylo-hexose and 4-azido-4-deoxy-D-glucose as potential inhibitors for lactose synthase [uridine 5'-(alpha-D-galactopyranosyl pyrophosphate):D-glucose 4-beta-D-galactopyranosyltransferase, EC 2.4.1.22]. These syntheses involved SN2 displacement of the 4-methylsulfonyloxy group of methyl 2,3,6-tri-O-benzoyl-4-O-methylsulfonyl-alpha-D-galactopyranoside by iodide and azide ions. In both cases, inversion
IrfanUllah Khan et al.
Pakistan journal of pharmaceutical sciences, 29(1), 213-219 (2016-02-02)
The 1,2,3-triazole-containing (1-azido-1-deoxy-β-D-glucopyranoside) complex was synthesized using click chemistry approach and evaluated its potential as a tumor-seeking agent. In the present study, (99m)Tc-tricarbonyl labeled (1-azido-1-deoxy-β-D-glucopyranoside) radiotracer [(99m)Tc(CO)(3)-BM], (where BM stands for biomolecule, e.g., (1-azido-1-deoxy-β-D- glucopyranoside)) was synthesized via click chemistry
André Luís Branco de Barros et al.
Bioorganic & medicinal chemistry letters, 19(9), 2497-2499 (2009-04-01)
A d-glucose-MAG(3) derivative was successfully synthesized and radiolabeled in high labeling yield. Biodistribution studies in Ehrlich tumor-bearing mice were performed. This compound showed high accumulation in tumor tissue with high tumor-to-muscle ratio and moderate tumor-to-blood ratio. Thus, d-glucose-MAG(3) is a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service