205338
Tellurium tetrachloride
99%
Synonym(s):
Tellurium chloride, Tellurium(IV) chloride, Tetrachlorotellurium
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
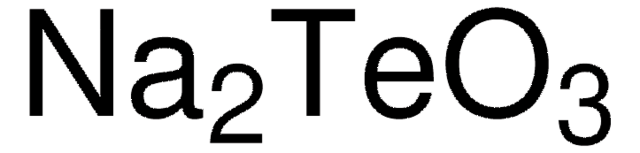
TeCl4
CAS Number:
Molecular Weight:
269.41
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Assay:
99%
form:
powder, crystals or chunks
Recommended Products
Quality Level
Assay
99%
form
powder, crystals or chunks
bp
380 °C (lit.)
mp
224 °C (lit.)
density
3.26 g/mL at 25 °C (lit.)
SMILES string
Cl[Te](Cl)(Cl)Cl
InChI
1S/Cl4Te/c1-5(2,3)4
InChI key
SWLJJEFSPJCUBD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Atomic number of base material: 52 Tellurium
Application
- Highly efficient regioselective synthesis of organotellurium compounds: Explores the reactions of Tellurium Tetrachloride with 1-alkenes, highlighting its utility in organic synthesis (VA Potapov et al., 2017).
- Cyclization Reactions: TeCl4 acts as a bis-electrophile in double-electrophilic cyclization reactions, demonstrating its versatility in organic transformations (N Korol, M Slivka, 2018).
- Regio-and stereoselective addition of tellurium tetrachloride: Investigates the addition of TeCl4 to methyl propargyl ether, emphasizing the stereochemistry of the addition process (MV Musalova et al., 2017).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Maria V Musalova et al.
Molecules (Basel, Switzerland), 17(5), 5770-5779 (2012-05-17)
The reaction of tellurium tetrachloride with acetylene proceeds in a stereospecific anti-addition manner to afford the novel products E-2-chlorovinyltellurium trichloride and E,E-bis(2-chlorovinyl)tellurium dichloride. Reaction conditions for the selective preparation of each of these products were found. The latter was obtained
Shalini Roy et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 49(10), 2564-2574 (2011-07-12)
Tellurium tetrachloride (TeCl(4)) and diphenyl ditelluride (DPDT) cytotoxicity, was investigated in rat astrocytes. Concentrations of 0.24-250μM (24h) were tested for viability using MTT(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) and trypan blue exclusion. MTT showed significant decreases at all concentrations tested for both compounds.
Kyoko Iwase et al.
Environmental toxicology, 19(6), 614-619 (2004-11-05)
Despite the growing use of organotellurium compounds in the chemical and biomedical fields, there has been no great concern about their toxicity until now. To test the possibility that diphenyl ditelluride (DPDT) and tellurium chloride (TeCl2), organic and inorganic tellurium
J F Van Vleet et al.
American journal of veterinary research, 43(11), 2000-2009 (1982-11-01)
Seventy newly hatched ducklings were fed a commercial ration with 500 mg of added Te (as tetrachloride)/kg of feed for up to 28 days. Ducklings were euthanatized at day 14, 21, and 28, the hearts were studied by gross, microscopic
Puneet Vij et al.
Environmental toxicology and pharmacology, 34(3), 768-782 (2012-10-17)
Diphenyl ditelluride (DPDT) and tellurium tetrachloride (TeCl(4)) were evaluated for toxicity in transformed (HT-29, Caco-2) and non-transformed colon cells (CCD-18Co). Significant decreases in viability were observed with DPDT exposure in HT-29 (62.5-1000 μM), Caco-2 (31.25-1000 μM) and CCD-18Co cells (500-1000
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service