148113
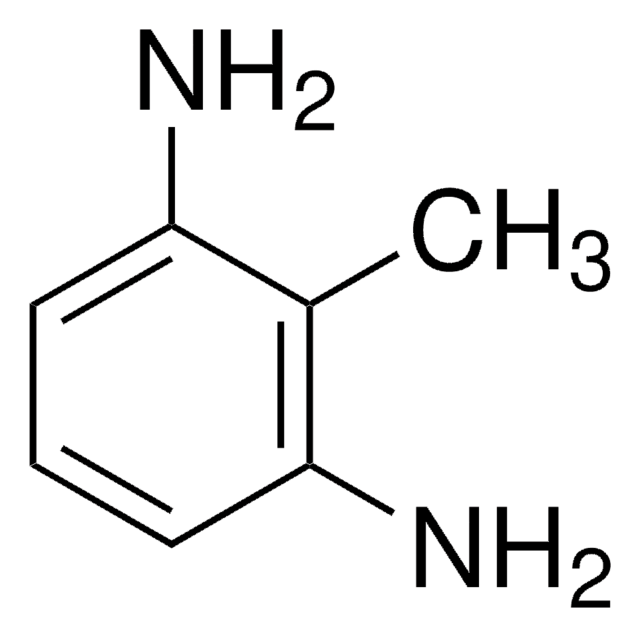
2,6-Diaminotoluene
97%
Synonym(s):
2,6-Toluenediamine, 2,6-Tolylenediamine, 2-Methyl-m-phenylenediamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C6H3(NH2)2
CAS Number:
Molecular Weight:
122.17
Beilstein:
2079476
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
solid
mp
104-106 °C (lit.)
SMILES string
Cc1c(N)cccc1N
InChI
1S/C7H10N2/c1-5-6(8)3-2-4-7(5)9/h2-4H,8-9H2,1H3
InChI key
RLYCRLGLCUXUPO-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2 - Muta. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Differential in vivo mutagenicity of the carcinogen/non-carcinogen pair 2,4- and 2,6-diaminotoluene.
J J Hayward et al.
Carcinogenesis, 16(10), 2429-2433 (1995-10-01)
The aromatic amines 2,4-diaminotoluene (2,4-DAT) and 2,6-diaminotoluene (2,6-DAT) are structural isomers that have been extensively studied for their mutagenic and carcinogenic characteristics. Both compounds are equally mutagenic in the Ames/Salmonella assay in the presence of S9. However, the differences in
M Taningher et al.
Toxicology, 99(1-2), 1-10 (1995-05-05)
Among aminoaromatics, 2,4-diaminotoluene (2,4-DAT) and 2,6-diaminotoluene (2,6-DAT) represent a conflicting couple of isomers; despite showing the same structural alert to DNA reactivity (and thus potential genotoxicity), they are different in terms of carcinogenicity. Of the two, 2,4-DAT alone is a
M L Cunningham et al.
Environmental health perspectives, 104 Suppl 3, 683-686 (1996-05-01)
The aromatic amines 2,4-diaminotoluene (2,4-DAT) and 2,6-diaminotoluene (2,6-DAT) are structural isomers that have been extensively studied for their mutagenic and carcinogenic characteristics. Both compounds are rapidly absorbed after oral administration and are equally mutagenic in the Ames test; however, 2,4-DAT
P Lind et al.
The Analyst, 122(1), 51-56 (1997-01-01)
Blood and urine samples were collected from six workers and two volunteers exposed to thermal degradation products from toluene diisocyanate (TDI)-based polyurethane (PUR) before and during the summer vacation. Air samples were collected on filters impregnated with 9-(N-methylaminomethyl)anthracene. The concentrations
Diaminotoluenes induce intrachromosomal recombination and free radicals in Saccharomyces cerevisiae.
R J Brennan et al.
Mutation research, 381(2), 251-258 (1998-01-22)
The carcinogenicity of aniline-based aromatic amines is poorly reflected by their activity in short-term mutagenicity assays such as the Salmonella typhimurium reverse mutation (Ames) assay. More information about the mechanism of action of such carcinogens is needed. Here we report
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service