144436
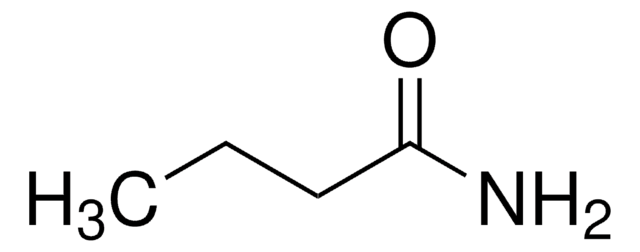
Isobutyramide
99%
Synonym(s):
2-Methylpropionamide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)2CHCONH2
CAS Number:
Molecular Weight:
87.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
bp
216-220 °C (lit.)
mp
127-131 °C (lit.)
density
1.013 g/mL at 25 °C (lit.)
functional group
amide
SMILES string
CC(C)C(N)=O
InChI
1S/C4H9NO/c1-3(2)4(5)6/h3H,1-2H3,(H2,5,6)
InChI key
WFKAJVHLWXSISD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Isobutyramide was used for chemical grafting of human serum albumin during the synthesis of sequentialy assembled protein capsules.
Biochem/physiol Actions
Isobutyramide activates transcription of human gamma-globin gene and murine embryonic epsilon(y)-globin gene. It is useful in the treatment of β-thalassemia and sickle cell disease.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M E Gleave et al.
Journal of cellular biochemistry, 69(3), 271-281 (1998-05-15)
Progression to androgen independence remains the main obstacle to improving survival and quality of life in patients with advanced prostate cancer. Induction of differentiation may serve as a rational basis for prevention of progression to androgen independence by modulating gene
Thermosensitive molecular assemblies from poly(amidoamine) dendron-based lipids.
Kenji Kono et al.
Angewandte Chemie (International ed. in English), 50(28), 6332-6336 (2011-05-21)
S Reich et al.
Blood, 96(10), 3357-3363 (2000-11-09)
The butyrate derivative isobutyramide (IBT) increases fetal hemoglobin (HbF) in patients with beta-hemoglobinopathies, but little is known about its usefulness for prolonged therapeutic use. We treated 8 patients with transfusion-dependent beta-thalassemia with 350 mg/kg of body weight per day of
H Miyake et al.
International journal of cancer, 93(1), 26-32 (2001-06-08)
Sodium butyrate (NaBt), a physiologically occurring short-chain fatty acid, induces differentiation as well as apoptosis in numerous cell types, and this induction is partially regulated by Bcl-2 expression. The objectives of our study were to characterize the in vitro effects
G B Strambini et al.
Biochemistry, 29(1), 203-208 (1990-01-09)
The phosphorescence properties of liver alcohol dehydrogenase from horse were characterized at limiting concentrations of coenzyme and coenzyme analogues. The emission decay kinetics of Trp-314 in strong, slowly exchanging, ternary complexes with NADH/isobutyramide, NAD/pyrazole, and NADH/dimethyl sulfoxide displays a markedly
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service