All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H8N2
CAS Number:
Molecular Weight:
144.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
125-128 °C (lit.)
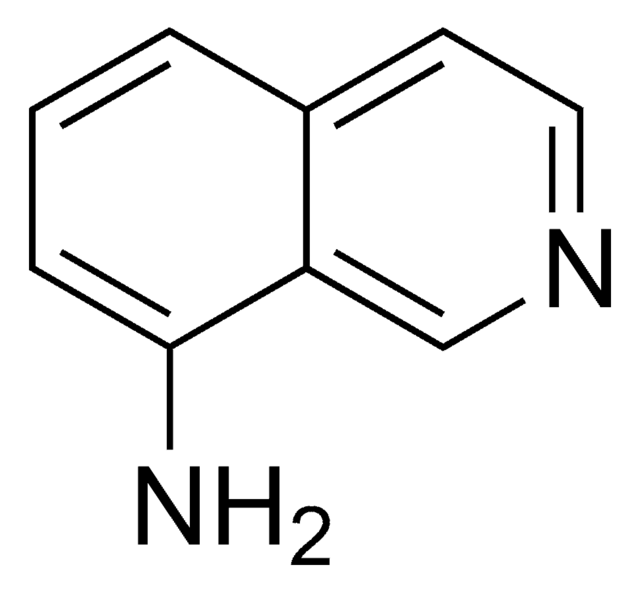
SMILES string
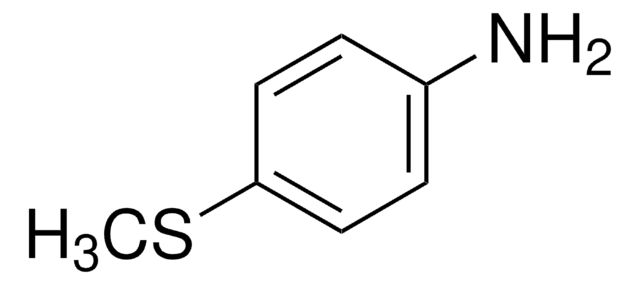
Nc1cccc2cnccc12
InChI
1S/C9H8N2/c10-9-3-1-2-7-6-11-5-4-8(7)9/h1-6H,10H2
InChI key
DTVYNUOOZIKEEX-UHFFFAOYSA-N
General description
5-Aminoisoquinoline forms 1:1 host−guest inclusion complex with β-cyclodextrin. It enhances the chemiluminescence of luminol-H2O2-horseradish peroxidase.
Application
5-Aminoisoquinoline (5AIQ) was used to study the effect of addition of β-cyclodextrin on the absorption and emission properties of 5AIQ.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Relation between the structure of some heterocyclic derivatives and other compounds, and their effects as enhancers or inhibitors of the luminol-H2O2-horseradish peroxidase chemiluminescence.
Garcia SF, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 105(!), 11-14 (1997)
Jürgen Bosch et al.
Journal of medicinal chemistry, 49(20), 5939-5946 (2006-09-29)
The 1.8 A resolution de novo structure of nucleoside 2-deoxyribosyltransferase (EC 2.4.2.6) from Trypanosoma brucei (TbNDRT) has been determined by SADa phasing in an unliganded state and several ligand-bound states. This enzyme is important in the salvage pathway of nucleoside
Host-guest complexation between 5-aminoisoquinoline and ?-cyclodextrin and its effect on spectral and prototropic characteristics.
Rajamohan R, et al.
Journal of Inclusion Phenomena and Macrocyclic Chemistry, 73(1-4), 99-108 (2012)
Sheikh Fayaz Ahmad et al.
Molecular immunology, 63(2), 394-405 (2014-10-12)
Increasing indication is unveiling a role for poly(ADP-ribose) polymerase (PARP)-1 in the regulation of inflammatory/immune responses. The aim of the present study was to determine the potential anti-inflammatory effects of PARP-1 inhibitor 5-aminoisoquinolinone (5-AIQ) to explore the role of PARP-1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service