C65408
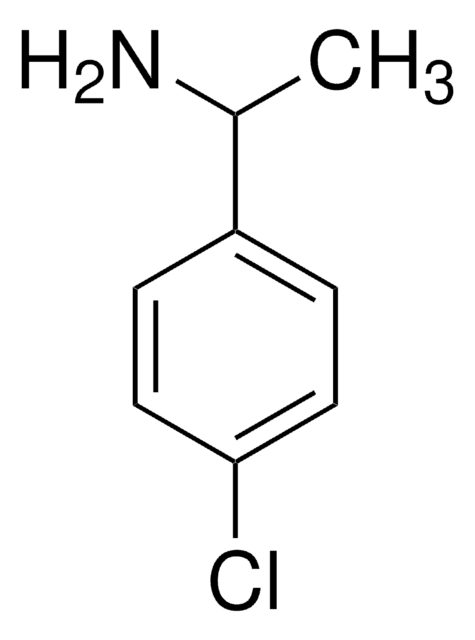
2-(4-Chlorophenyl)ethylamine
98%
Synonym(s):
4-Chlorophenethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC6H4CH2CH2NH2
CAS Number:
Molecular Weight:
155.62
Beilstein:
508247
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.548 (lit.)
bp
60-65 °C/0.1 mmHg (lit.)
density
1.112 g/mL at 25 °C (lit.)
SMILES string
NCCc1ccc(Cl)cc1
InChI
1S/C8H10ClN/c9-8-3-1-7(2-4-8)5-6-10/h1-4H,5-6,10H2
InChI key
SRXFXCKTIGELTI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
222.8 °F - closed cup
Flash Point(C)
106 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Khalid Touiki et al.
Psychopharmacology, 182(4), 562-569 (2005-09-01)
Harmane and norharmane (two beta-carbolines) are tobacco components or products. The effects of harmane and norharmane on serotonergic raphe neurons remain unknown. Harmane and norharmane are inhibitors of the monoamine oxidases A (MAO-A) and B (MAO-B), respectively. To study the
Giulia Guiducci et al.
Nucleic acids research, 47(8), 4240-4254 (2019-02-28)
Enzymes of intermediary metabolism are often reported to have moonlighting functions as RNA-binding proteins and have regulatory roles beyond their primary activities. Human serine hydroxymethyltransferase (SHMT) is essential for the one-carbon metabolism, which sustains growth and proliferation in normal and
D Becquet et al.
Neuroscience, 39(3), 639-647 (1990-01-01)
Using a push-pull cannula technique and an isotopic method for estimating [3H]serotonin continuously synthesized from [3H]tryptophan, the effects of changes in the release of serotonin in the dorsalis raphe nucleus on in vivo release of [3H]serotonin in the cat caudate
Marina Marani et al.
Oncotarget, 7(4), 4570-4583 (2015-12-31)
Serine hydroxymethyltransferase (SHMT) is a central enzyme in the metabolic reprogramming of cancer cells, providing activated one-carbon units in the serine-glycine one-carbon metabolism. Previous studies demonstrated that the cytoplasmic isoform of SHMT (SHMT1) plays a relevant role in lung cancer.
G B Baker et al.
Progress in neuro-psychopharmacology & biological psychiatry, 6(4-6), 343-346 (1982-01-01)
1. The accumulation of p-chlorophenylethylamine (pCPE) in rat brain after administration of pargyline plus p-chlorophenylalanine (pCPA) is demonstrated. 2. Measurements of pCPE in brain were performed at 0.25 h, 1 h and 4 h after administration of pCPA to pargyline-pretreated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service