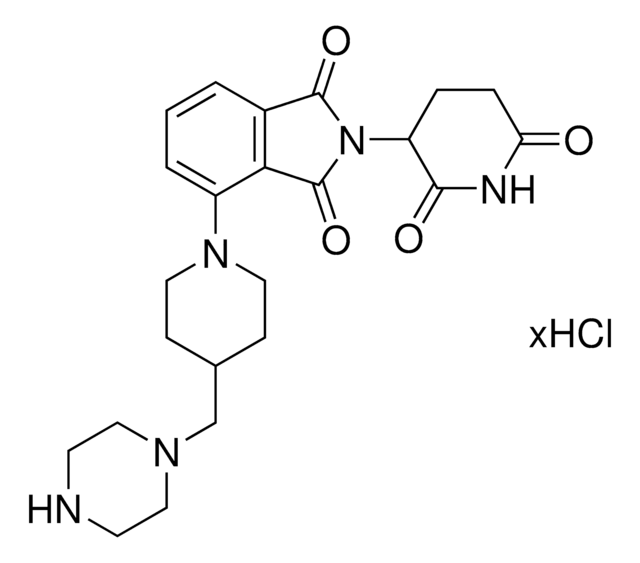
930571
Pomalidomide-piperidine-carboxylic acid
≥95%
Synonym(s):
1-[2-(2,6-Dioxo-3-piperidinyl)-2,3-dihydro-1,3-dioxo-1H-isoindol-5-yl], 1-[2-(2,6-Dioxo-3-piperidyl)-1,3-dioxo-isoindolin-5-yl]piperidine-4-carboxylic acid, 4-Piperidinecarboxylic acid
About This Item
Recommended Products
ligand
pomalidomide
Quality Level
Assay
≥95%
form
powder
functional group
carboxylic acid
storage temp.
2-8°C
SMILES string
O=C(O)C1CCN(C2=CC=C3C(=O)N(C(=O)C3=C2)C4C(=O)NC(=O)CC4)CC1
Related Categories
Application
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Other Notes
Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O′PROTAC): Effective Targeting of LEF1 and ERG
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service