All Photos(1)
About This Item
Empirical Formula (Hill Notation):
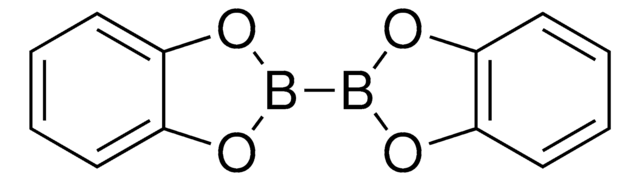
C12H24B2O4
CAS Number:
Molecular Weight:
253.94
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
mp
98-102 °C (lit.)
SMILES string
CC1CC(C)(C)OB(O1)B2OC(C)CC(C)(C)O2
InChI
1S/C12H24B2O4/c1-9-7-11(3,4)17-13(15-9)14-16-10(2)8-12(5,6)18-14/h9-10H,7-8H2,1-6H3
InChI key
UEBSWKNVDRJVHN-UHFFFAOYSA-N
Application
Bis(hexylene glycolato)diboron can be used as a reagent:
- To prepare aryl boronate esters via metal-catalyzed direct C-H borylation of aryl compounds.
- In the chemoselective synthesis of C−C coupling products via nickel catalyzed coupling of primary and secondary alkyl halides.
- In the nickel-catalyzed regioselective arylboration of terminal nonactivated alkenes to yield alkyl boranes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nickel-catalyzed cross-coupling of unactivated alkyl halides using bis (pinacolato) diboron as reductant
X Hailiang, et al.
Chemical Science, 4(10), 4022-4029 (2013)
Borylation of Arenes with Bis (hexylene glycolato) diboron
Liskey CW and Hartwig JF
Synthesis, 45(13), 1837-1842 (2013)
Nickel-Catalyzed Migratory Arylboration of Nonactivated Alkenes
Wang W, et al.
Synlett, 30(16), 1850-1854 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![BIS[(-)PINANEDIOLATO]DIBORON AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/148/156/0765f77a-05ac-45d3-a683-9eb03b355626/640/0765f77a-05ac-45d3-a683-9eb03b355626.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)