141712
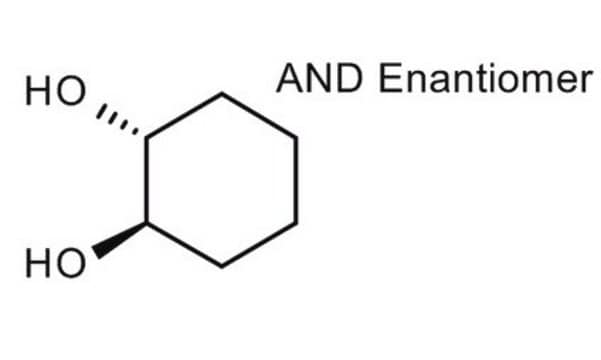
trans-1,2-Cyclohexanediol
98%
Synonym(s):
1,2-trans -Cyclohexanediol, 1,2-trans -Dihydroxycyclohexane, trans -2-Hydroxycyclohexanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H10(OH)2
CAS Number:
Molecular Weight:
116.16
Beilstein:
3193810
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
form
solid
mp
101-104 °C (lit.)
SMILES string
O[C@@H]1CCCC[C@H]1O
InChI
1S/C6H12O2/c7-5-3-1-2-4-6(5)8/h5-8H,1-4H2/t5-,6-/m1/s1
InChI key
PFURGBBHAOXLIO-PHDIDXHHSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J Mráz et al.
Scandinavian journal of work, environment & health, 25(3), 233-237 (1999-08-18)
This study explored the acute effect of ethanol (EtOH) on the urinary excretion of cyclohexanol (CH-ol), 1,2- and 1,4-cyclohexanediol (CH-diol), biomarkers of exposure to important solvents, and chemical intermediates cyclohexanone (CH-one), cyclohexane (CH) and cyclohexanol. Volunteers (5-8 in each group)
Roosmarijn E Vandenbroucke et al.
Nucleic acids research, 35(12), e86-e86 (2007-06-23)
One of the major obstacles in non-viral gene transfer is the nuclear membrane. Attempts to improve the transport of DNA to the nucleus through the use of nuclear localization signals or importin-beta have achieved limited success. It has been proposed
Yoshihito Shiota et al.
Inorganic chemistry, 50(13), 6200-6209 (2011-06-04)
The catalytic conversion of 1,2-cyclohexanediol to adipic anhydride by Ru(IV)O(tpa) (tpa ═ tris(2-pyridylmethyl)amine) is discussed using density functional theory calculations. The whole reaction is divided into three steps: (1) formation of α-hydroxy cyclohexanone by dehydrogenation of cyclohexanediol, (2) formation of
David K Breslow et al.
The Journal of cell biology, 203(1), 129-147 (2013-10-09)
Specific proteins are concentrated within primary cilia, whereas others remain excluded. To understand the mechanistic basis of entry into cilia, we developed an in vitro assay using cells in which the plasma membrane was permeabilized, but the ciliary membrane was
M Shahjahan Kabir et al.
The Journal of organic chemistry, 75(11), 3626-3643 (2010-05-01)
cis-1,2-Cyclohexanediol (L3) has been shown to be an efficient and versatile bidentate O-donor ligand that provides a highly active Cu-catalytic system. It was more effective than diols such as trans-1,2-cyclohexanediol or ethylene glycol. This commercially available cis-1,2-cyclohexanediol ligand facilitated the
Chromatograms
suitable for GCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service