31571
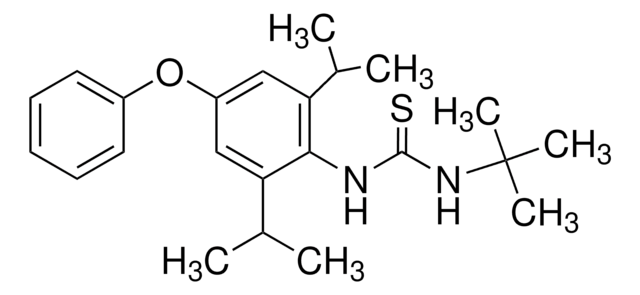
Diafenthiuron
PESTANAL®, analytical standard
Synonym(s):
1-tert-Butyl-3-(2,6-diisopropyl-4-phenoxyphenyl)-thiourea, N-[2,6-Bis(1-methylethyl)-4-phenoxyphenyl]-N′-(1,1-dimethylethyl)-thiourea
About This Item
Recommended Products
grade
analytical standard
Quality Level
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
agriculture
environmental
format
neat
SMILES string
CC(C)c1cc(Oc2ccccc2)cc(C(C)C)c1NC(=S)NC(C)(C)C
InChI
1S/C23H32N2OS/c1-15(2)19-13-18(26-17-11-9-8-10-12-17)14-20(16(3)4)21(19)24-22(27)25-23(5,6)7/h8-16H,1-7H3,(H2,24,25,27)
InChI key
WOWBFOBYOAGEEA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Cotton and groundnut oil by quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction procedure, low-temperature freezing and dispersive clean-up followed by quantification using gas chromatography (GC) equipped with electron capture detector (ECD) as well as flame photometric detector (FPD) and liquid chromatography-tandem mass spectrometry (LC-MS/MS).
- Tomatoes by QuEChERS extraction and LC combined with triple quadrupole MS/MS with electrospray ionization source (ESI).
- Fruit juice samples by ionic liquid-assisted liquid-phase microextraction based on the solidification of floating organic droplets (ILSFOD-LLME) and high performance liquid chromatography (HPLC) equipped with a variable-wavelength detector (VWD).
- Water and wastewater by solid phase extraction (SPE) and LC combined with time-of-flight (TOF) MS.
Recommended products
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
>300.2 °F - closed cup
Flash Point(C)
> 149 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service