All Photos(1)
About This Item
Linear Formula:
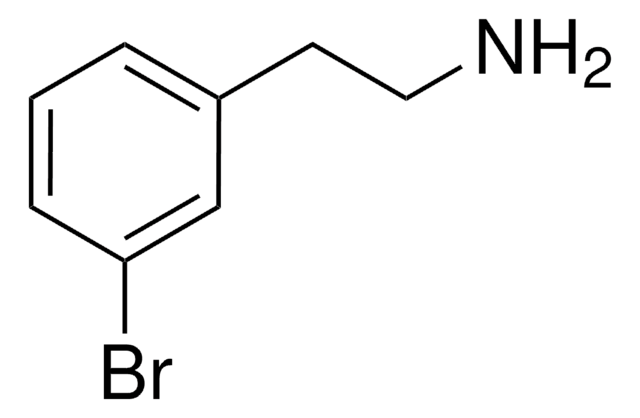
BrC6H3(OCH3)(CH2CH2NH2)
CAS Number:
Molecular Weight:
230.10
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D >1.5800 (lit.)
density
1.389 g/mL at 25 °C (lit.)
SMILES string
COc1ccc(CCN)cc1Br
InChI
1S/C9H12BrNO/c1-12-9-3-2-7(4-5-11)6-8(9)10/h2-3,6H,4-5,11H2,1H3
InChI key
JHVALSRTUOVNLL-UHFFFAOYSA-N
General description
3-Bromo-4-methoxyphenethylamine can be synthesized by reacting N-benzoyl-3-bromo-4-methoxyphenethylamine, acetic acid and HCl in the presence of N2.
Application
3-Bromo-4-methoxyphenethylamine (3-Bromo-4-methoxy-β-phenethylamine) may be used as a starting reagent in the synthesis of 3-bromo-4-methoxy-5-nitro-β-phenethylamine. It may also be used to synthesize 4-bromo-N-(3-bromo-4-methoxyphenethyl)-1H-pyrrole-2-carboxamide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Total synthesis and biological evaluation of the marine bromopyrrole alkaloid dispyrin: elucidation of discrete molecular targets with therapeutic potential.
Kennedy JP, et al.
Journal of Natural Products, 1783-1786 (2008)
Synthesis of Aminoisoquinolines and Related Compounds. X. A Modified Synthesis of dl-Cularine.
Ishiwata S, et al.
Chemical & Pharmaceutical Bulletin, 18(9), 1850-1855 (1970)
Kumar Saurav et al.
Marine drugs, 18(2) (2020-02-26)
Marine sponges, a well-documented prolific source of natural products, harbor highly diverse microbial communities. Their extracts were previously shown to contain quorum sensing (QS) signal molecules of the N-acyl homoserine lactone (AHL) type, known to orchestrate bacterial gene regulation. Some
Syntheses of the marine metabolites verongamine, hemibastadin-2, and aerothionin using the cyano ylide coupling methodology.
Wasserman HH and Wang J.
The Journal of Organic Chemistry, 63(16), 5581-5586 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service