All Photos(1)
About This Item
Linear Formula:
HOCH2CH2CH[NHCO2C(CH3)3]CH2OH
CAS Number:
Molecular Weight:
205.25
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
optical activity
[α]20/D −8°, c = 1 in chloroform
mp
65-69 °C (lit.)
functional group
amine
hydroxyl
SMILES string
CC(C)(C)OC(=O)N[C@H](CO)CCO
InChI
1S/C9H19NO4/c1-9(2,3)14-8(13)10-7(6-12)4-5-11/h7,11-12H,4-6H2,1-3H3,(H,10,13)/t7-/m0/s1
InChI key
KLRRFBSWOIUAHZ-ZETCQYMHSA-N
Application
(S)-(−)-2-(Boc-amino)-1,4-butanediol can be used as a reactant to synthesize:
- Thiourea-based organocatalysts for asymmetric Michael addition reactions of nitroalkenes to α-nitrocyclohexanone.
- Bis-copper (II) complex based catalysts for enantioselective Michael reactions.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Copper (II) in organic synthesis. XI. Evaluation of the ligand architecture on the efficiency of a copper (II) catalyst for enantioselective Michael reactions
Desimoni G, et al.
Tetrahedron, 51(14), 4131-4144 (1995)
Asymmetric Michael additions of α-nitrocyclohexanone to aryl nitroalkenes catalyzed by natural amino acid-derived bifunctional thioureas
Jo?rres M, et al.
Organic Letters, 14(17), 4518-4521 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service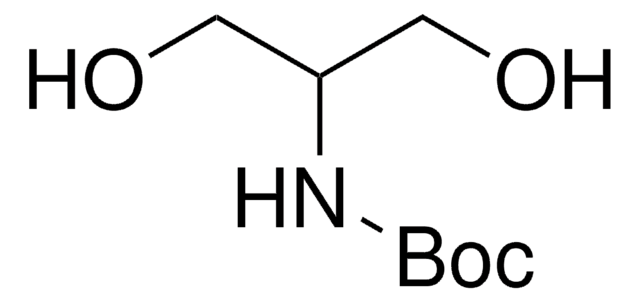



![Trimethylolpropane tris[poly(propylene glycol), amine terminated] ether average Mn 440](/deepweb/assets/sigmaaldrich/product/structures/186/658/1b1d510a-705a-4bfd-b90a-9dec80d64467/640/1b1d510a-705a-4bfd-b90a-9dec80d64467.png)