47316
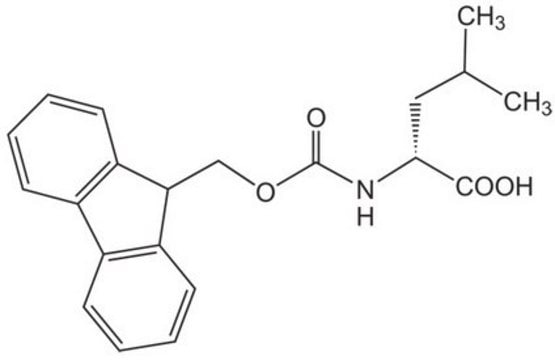
Fmoc-D-Leu-OH
≥95.0% (TLC)
Synonym(s):
Fmoc-D-leucine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C21H23NO4
CAS Number:
Molecular Weight:
353.41
Beilstein:
4299950
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Quality Level
Assay
≥95.0% (TLC)
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
2-8°C
SMILES string
CC(C)C[C@@H](NC(=O)OCC1c2ccccc2-c3ccccc13)C(O)=O
InChI
1S/C21H23NO4/c1-13(2)11-19(20(23)24)22-21(25)26-12-18-16-9-5-3-7-14(16)15-8-4-6-10-17(15)18/h3-10,13,18-19H,11-12H2,1-2H3,(H,22,25)(H,23,24)/t19-/m1/s1
InChI key
CBPJQFCAFFNICX-LJQANCHMSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zhimou Yang et al.
Chemical communications (Cambridge, England), (35)(35), 4414-4416 (2005-09-02)
Two types of therapeutic agents, which have discrete yet complementary functions, self-assemble into nanofibers in water to formulate a new supramolecular hydrogel as a self-delivery biomaterial to reduce the toxicity of uranyl oxide at the wound sites.
M J Miller et al.
The Journal of pharmacology and experimental therapeutics, 266(1), 468-472 (1993-07-01)
Anti-inflammatory properties have been ascribed to a series of N-(fluorenyl-9-methoxycarbonyl) amino acids called leumedins that inhibit the activity of granulocytes and T-lymphocytes. We evaluated one of these leumedins, N-(fluorenyl-9-methoxycarbonyl) leucine (NPC 15199), in a model of ileitis in guinea pigs.
C R Jan et al.
The Chinese journal of physiology, 43(1), 29-33 (2000-06-17)
This report demonstrates that NPC-15199 [(N-(9-fluorenylmethoxycarbonyl)L-leucine)], a novel anti-inflammatory agent, increases intracellular Ca2+ concentration ([Ca2+]i) in human bladder female transitional cancer (BFTC) cells. Using fura-2 as a Ca2+ probe, NPC-15199 (0.1-2 mM) was found to increase [Ca2+]i concentration-dependently. The response
G Cheng et al.
Langmuir : the ACS journal of surfaces and colloids, 26(7), 4990-4998 (2010-01-16)
The self-assembly and hydrogelation properties of two Fmoc-tripeptides [Fmoc = N-(fluorenyl-9-methoxycarbonyl)] are investigated, in borate buffer and other basic solutions. A remarkable difference in self-assembly properties is observed comparing Fmoc-VLK(Boc) with Fmoc-K(Boc)LV, both containing K protected by N(epsilon)-tert-butyloxycarbonate (Boc). In
Chung-Ren Jan et al.
The Chinese journal of physiology, 45(3), 117-122 (2003-06-24)
In Madin-Darby canine kidney (MDCK) cells, effect of NPC-15199 on intracellular Ca2+ concentration ([Ca2+]i) was investigated by using fura-2. NPC-15199 (100-1000 microM) caused a rapid and sustained increase of [Ca2+]i in a concentration-dependent manner (EC50=500 microM). NPC-15199-induced [Ca2+]i rise was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service