SML3713
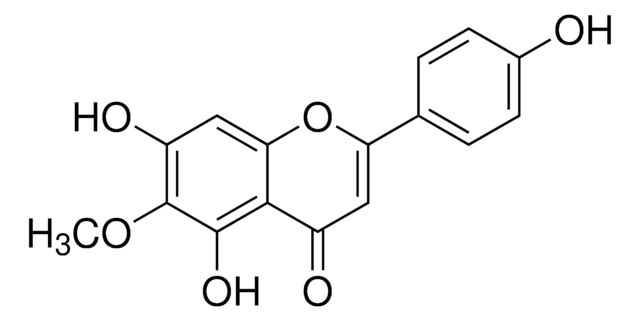
HM-chromanone
≥98% (HPLC)
Synonym(s):
(3E)-2,3-Dihydro-5-hydroxy-3-[(2-hydroxyphenyl)methylene]-7-methoxy-4H-1-benzopyran-4-one, (E)-5-hydroxy-7-methoxy-3-(20-hydroxybenzyl)-4-chromanone, Portulacanone D
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C17H14O5
CAS Number:
Molecular Weight:
298.29
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 2 mg/mL, clear
storage temp.
2-8°C
Biochem/physiol Actions
Sappanine-type homoisoflavonoid, isolated from Portulaca oleracea (purslane) that exhibits anti-diabetic effects.
HM-chromanone is a sappanine-type homoisoflavonoid, isolated from Portulaca oleracea (purslane). HM-chromanone exhibits anti-diabetic effects and inhibits adipogenesis by regulating AMPK activity in adipocytes. HM-chromanone potently activates AMPK in hepatocytes, thus suppressing hepatic glucose production. HM-chromanone does not display significant toxicity.
HM-chromanone is a sappanine-type homoisoflavonoid, isolated from Portulaca oleracea (purslane). HM-chromanone exhibits anti-diabetic effects and inhibits adipogenesis by regulating AMPK activity in adipocytes. HM-chromanone potently activates AMPK in hepatocytes, thus suppressing hepatic glucose production. HM-chromanone does not display significant toxicity.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
HM-Chromanone Ameliorates Hyperglycemia and Dyslipidemia in Type 2 Diabetic Mice
Nutrients, 14(9), 1951-1951 (2022)
HM-Chromanone Isolated from Portulaca oleracea L. Protects INS-1 Pancreatic ? Cells against Glucotoxicity-Induced Apoptosis
Nutrients, 11(2), 404-404 (2019)
HM-chromanone suppresses hepatic glucose production via activation of AMP-activated protein kinase in HepG2 cell
European Journal of Pharmacology, 928, 175108-175108 (2022)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service