M1296
Malonic acid
ReagentPlus®, 99%
Synonym(s):
Propanedioic acid
Sign Into View Organizational & Contract Pricing
All Photos(6)
About This Item
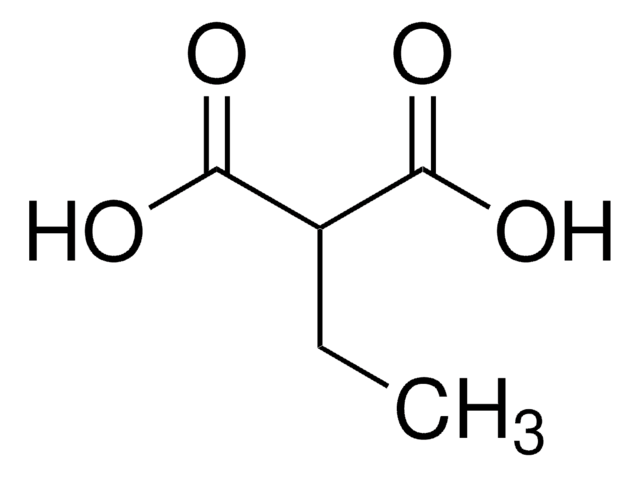
Linear Formula:
CH2(COOH)2
CAS Number:
Molecular Weight:
104.06
Beilstein:
1751370
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
form
chunks
granular
powder or crystals
mp
132-135 °C (dec.) (lit.)
solubility
1 M NaOH: 100 mg/mL, clear to slightly hazy, colorless to faintly yellow
SMILES string
OC(=O)CC(O)=O
InChI
1S/C3H4O4/c4-2(5)1-3(6)7/h1H2,(H,4,5)(H,6,7)
InChI key
OFOBLEOULBTSOW-UHFFFAOYSA-N
Gene Information
human ... SRC(6714)
Looking for similar products? Visit Product Comparison Guide
General description
Malonic acid (MA), also known as propanedioic acid, is a dicarboxylic acid. The crystals of MA are triclinic at room temperature. The oxidation of malonic acid by cerium (IV) in sulfuric acid solution has been studied. The reaction kinetics of the photocatalytic decomposition of MA in aqueous suspensions of titanium dioxide (TiO2) have been described.
Application
Malonic acid may be used as a cross-linking agent between corn starch and potato starch to improve its mechanical properties.
Other Notes
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Redi-Dri is a trademark of Sigma-Aldrich Co. LLC
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
314.6 °F - closed cup
Flash Point(C)
157 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
On the oxidation of malonic acid by ceric ions.
Barkin S, et al.
International Journal of Chemical Kinetics, 10(6), 619-636 (1978)
The crystal structure of malonic acid.
Goedkoop JA and MacGillavry CH.
Acta Crystallographica, 10(2), 125-127 (1957)
Photocatalytic degradation of malonic acid in aqueous suspensions of titanium dioxide: an initial kinetic investigation of CO2 photogeneration.
Inel Y and Okte AN.
Journal of Photochemistry and Photobiology A: Chemistry, 96(1), 175-180 (1996)
Refinement of the crystal structure of malonic acid, C3H4O4.
Jagannathan NR, et al.
Journal of Chemical Crystallography, 24(1), 75-78 (1994)
Anna Shevchenko et al.
International journal of pharmaceutics, 436(1-2), 403-409 (2012-07-04)
Cocrystallization and salt formation have been shown to entail substantial promise in tailoring the physicochemical properties of drug compounds, in particular, their dissolution and hygroscopicity. In this work, we report on the preparation and comparative evaluation of a new cocrystal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service