T-049
Tacrolimus solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in acetonitrile
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−70°C
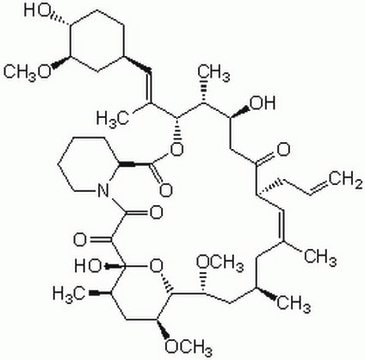
SMILES string
CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]2OC(=O)[C@@H]3CCCCN3C(=O)C(=O)[C@]4(O)O[C@H]([C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)OC)[C@H](C[C@H]4C)OC
InChI
1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1
InChI key
QJJXYPPXXYFBGM-LFZNUXCKSA-N
Gene Information
human ... FKBP1A(2280)
General description
Application
- Forensic toxicology and clinical implications: An analysis of AB-PINACA′s metabolites in solid tissues from an abuser, compared with in vitro experiments, highlights the compound′s potent effects and underscores its relevance in toxicological and forensic investigations (Minakata et al., 2021).
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
35.6 °F - closed cup
Flash Point(C)
2 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service