All Photos(1)
About This Item
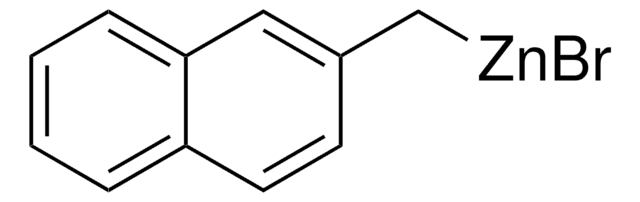
Empirical Formula (Hill Notation):
C4H3BrSZn
CAS Number:
Molecular Weight:
228.43
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
concentration
0.5 M in THF
density
0.973 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
Br[Zn]c1cccs1
InChI
1S/C4H3S.BrH.Zn/c1-2-4-5-3-1;;/h1-3H;1H;/q;;+1/p-1
InChI key
KBBMRTYVFOYNIW-UHFFFAOYSA-M
Related Categories
Application
2-Thienylzinc bromide can be used as:
- A starting material in the synthesis of heteroaryl sulfonamides by reacting with 2,4,6-trichlorophenyl chlorosulfate.
- A substrate in the synthesis of naphthacenodithiophene derived polymers, which are used in transistor and solar cell devices.
- An intermediate in the preparation of isoquinoline derived AICARFT inhibitors.
Legal Information
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-0.0 °F - closed cup
Flash Point(C)
-17.78 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of Heteroaryl Sulfonamides from Organozinc Reagents and 2, 4, 6-Trichlorophenyl Chlorosulfate
Colombe JR, et al.
Organic Letters, 17(12), 3170-3173 (2015)
Naphthacenodithiophene based polymers-new members of the acenodithiophene family exhibiting high mobility and power conversion efficiency
Knall A-C, et al.
Advances in Functional Materials, 26(38), 6961-6969 (2016)
Discovery of N-(6-Fluoro-1-oxo-1, 2-dihydroisoquinolin-7-yl)-5-[(3 R)-3-hydroxypyrrolidin-1-yl] thiophene-2-sulfonamide (LSN 3213128), a Potent and Selective Nonclassical Antifolate Aminoimidazole-4-carboxamide Ribonucleotide Formyltransferase (AICARFT) Inhibitor Effective at Tumor Suppression in a Cancer Xenograft Model
Fales KR, et al.
Journal of Medicinal Chemistry, 60(23), 9599-9616 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)