316539
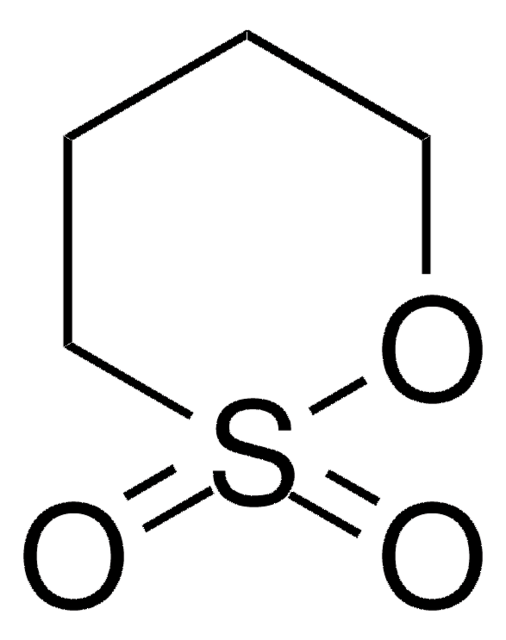
1,8-Naphthosultone
98%
Synonym(s):
1-Naphthol-8-sulfonic acid sultone, 8-Hydroxynaphthalene-1-sulfonic acid sultone
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H6O3S
CAS Number:
Molecular Weight:
206.22
Beilstein:
9381
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
154-157 °C (dec.) (lit.)
functional group
sulfonic acid
SMILES string
O=S1(=O)Oc2cccc3cccc1c23
InChI
1S/C10H6O3S/c11-14(12)9-6-2-4-7-3-1-5-8(13-14)10(7)9/h1-6H
InChI key
IEIADDVJUYQKAZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
DNA adducts of lactones, sultones, acylating agents and acrylic compounds.
J J Solomon
IARC scientific publications, (125)(125), 179-198 (1994-01-01)
Donald C Craig et al.
Carbohydrate research, 346(6), 854-857 (2011-03-11)
Acetolysis of methyl 5,6-di-O-acetyl-2,3-O-isopropylidene-β-L-gulofuranoside has yielded a sultone, 4-(1,2,5,6-tetra-O-acetyl-β-L-gulofuranos-3-yl)-6-methyl-1,2-oxathiin 2,2-dioxide (2) whose structure was determined by X-ray diffraction. (1)H and (13)C NMR spectral properties of 2 are presented together with a rationale for its formation. Preparation and properties of the
The derivation of quantitative correlations between skin sensitisation and physio-chemical parameters for alkylating agents, and their application to experimental data for sultones.
D W Roberts et al.
Journal of theoretical biology, 99(4), 807-825 (1982-12-21)
Sébastien Schmitt et al.
Chemical communications (Cambridge, England), 47(41), 11465-11467 (2011-09-29)
Sultones were subject to ring opening by nucleophilic attack with [(18)F]fluoride to afford easily purified (18)F-labelled hydrophilic sulfonated products in high yields. A two-step sequence including radiofluorination and coupling to lysine was then developed from a bis-sultone precursor as a
E Meschkat et al.
Chemical research in toxicology, 14(1), 118-126 (2001-02-15)
3-[(13)C]- and 2-[(13)C]hex-1-ene-1,3-sultones (1a and 1b, respectively) and 3-[(13)C]hex-1-ene-1,3-sultone 2a were incubated with human serum albumin in phosphate buffer at pH 8.1. In both cases, the main reaction was a hydrolysis via an S(N) reaction at position 3, but several
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service