All Photos(2)
About This Item
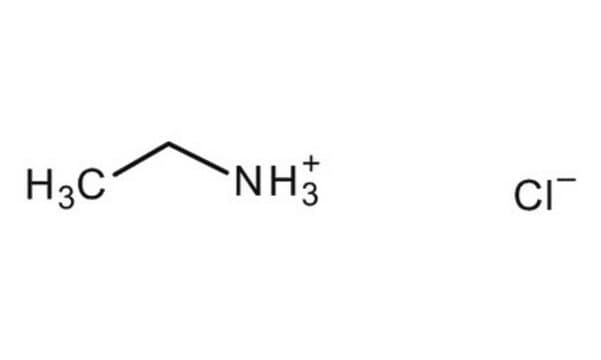
Linear Formula:
(C2H5)2NH · HBr
CAS Number:
Molecular Weight:
154.05
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
218-220 °C (lit.)
SMILES string
Br[H].CCNCC
InChI
1S/C4H11N.BrH/c1-3-5-4-2;/h5H,3-4H2,1-2H3;1H
InChI key
AATGHKSFEUVOPF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Diethylamine hydrobromide was used in the synthesis of α-bromovinyltrimethylsilane.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
SYNTHESES AND POLYMERIZATION OF α-TRIMETHYLSILYL ACRYLIC MONOMERS.
Canadian Journal of Chemistry, 41(12), 2977- 2982 (1963)
Spencer S Ericksen et al.
The Journal of pharmacology and experimental therapeutics, 342(2), 472-485 (2012-05-17)
In an effort to delineate how specific molecular interactions of dopamine receptor ligand classes vary between D2-like dopamine receptor subtypes, a conserved threonine in transmembrane (TM) helix 7 (Thr7.39), implicated as a key ligand interaction site with biogenic amine G
V M Gun'ko et al.
Journal of colloid and interface science, 348(2), 546-558 (2010-07-14)
Adsorption of low-molecular adsorbates (nonpolar hexane, nitrogen, weakly polar acetonitrile, and polar diethylamine, triethylamine, and water) onto individual (silica, alumina, titania), binary (silica/alumina (SA), silica/titania (ST)), and ternary (alumina/silica/titania, AST) fumed oxides was studied to analyse the effects of morphology
Wen-Hsien Tsai et al.
Journal of chromatography. A, 1217(3), 250-255 (2009-12-05)
A salting-out assisted liquid extraction coupled with back-extraction by a water/acetonitrile/dichloromethane ternary component system combined with high-performance liquid chromatography with diode-array detection (HPLC-DAD) was developed for the extraction and determination of sulfonamides in solid tissue samples. After the homogenization of
Junzo Hirano et al.
Journal of fluorescence, 20(2), 615-624 (2010-01-29)
A novel fluorescence enhancement-type derivatizing reagent for amino compounds, 6,7-difluoro-1,4-dihydro-1-methyl-4-oxo-3-quinolinecarboxylic acid (FMQC), was developed. FMQC reacts with aliphatic primary amino compounds to afford strong fluorescent derivatives having high photo-and thermo-stabilities. The FMQC derivatives of amino compounds showed 12-159 times higher
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service