MAB1012
Anti-Alkaline Phosphatase Antibody, E. coli, bacterial only
ascites fluid, Chemicon®
Synonym(s):
AP, Alk. Phos.
About This Item
Recommended Products
biological source
mouse
Quality Level
antibody form
ascites fluid
antibody product type
primary antibodies
clone
monoclonal
species reactivity
E. coli
manufacturer/tradename
Chemicon®
technique(s)
immunocytochemistry: suitable
immunoprecipitation (IP): suitable
western blot: suitable
isotype
IgG2a
UniProt accession no.
shipped in
dry ice
target post-translational modification
unmodified
Gene Information
Escherichia coli ... PhoA(945041)
Specificity
Immunogen
Application
Immunocytochemistry: reacts with E.coli. AP fusion protein targets in acetone fixed cell preparations. 1:4000, other fixatives or conditions untested.
ASSAY:
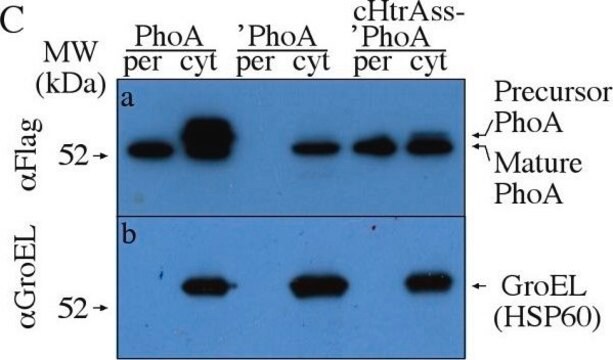
Preparation of E. coli TnphoA transformants: E. coli strain CC118 was transformed with plasmid pGEM-3Z containing TnphoA insertional mutations in the p101 gene of Mycoplasma hyorhinis, which encodes a protein with a typical N-terminal prokaryotic single peptide (Yogev et al. 1991).
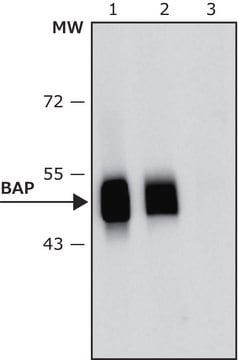
Identification of fusion protein with MAB1012 transformants: Transformants are grown in 2XYT medium to OD600=0.6. Cells were centrifuged 3 minutes at 10,000 x g, suspended in SDS-PAGE sample buffer, heated at 100°C for 5 minutes, frozen and thawed and centrifuged as above at room temperature to remove insoluble material. The sample is applied at 9% to a SDS-PAGE gel, and Western immunoblot is performed as described (Yogev et al. 1991).
Immunoprecipitation: 5μL of antibody per 500μL of lysate in RIPA or 0.5% triton X-100 solutions.
Optimal working dilutions must be determined by end user.
Physical form
Legal Information
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service