8.52110
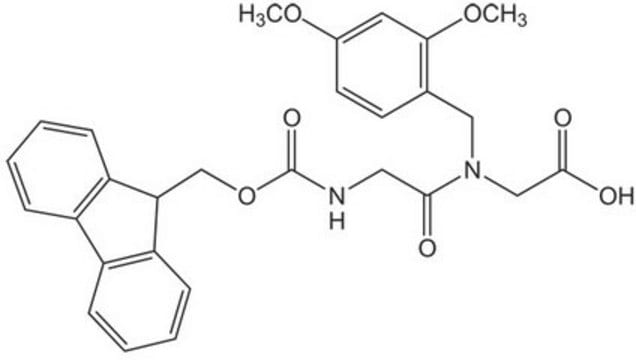
Fmoc-(Dmb)Gly-OH
Novabiochem®
Synonym(s):
Fmoc-(Dmb)Gly-OH, N-α-Fmoc-N-α-(2, 4-dimethoxybenzyl)-glycine
About This Item
Recommended Products
Quality Level
product line
Novabiochem®
Assay
≥94.0% (acidimetric)
≥98% (TLC)
≥99.0% (HPLC)
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
-10 to -25°C
InChI
1S/C26H25NO6/c1-31-18-12-11-17(24(13-18)32-2)14-27(15-25(28)29)26(30)33-16-23-21-9-5-3-7-19(21)20-8-4-6-10-22(20)23/h3-13,23H,14-16H2,1-2H3,(H,28,29)
InChI key
UIDQSTVPYKMCEY-UHFFFAOYSA-N
General description
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Overcoming Aggregation in Fmoc SPPS
Literature references
[1] M. El Haddadi, et al. (2000) J. Pept. Sci., 6, 560 (2000) J. Pept. Sci., 6, 560.
[2] K. Jauch, et al. in "Peptides 1996: Proc. 24th European Peptide Symposium", R. Ramage and R. Epton (Eds), Mayflower Scientific Ltd., 1996, pp 497.
Linkage
Analysis Note
Appearance of substance (visual): powder
Identity (IR): passes test
Purity (TLC(157B)): ≥ 98 %
Assay (HPLC, area%): ≥ 99.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): passes test
Assay (acidimetric): ≥ 94.0 %
Water (K. F.): ≤ 1.00 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
The ease of assembly of a given peptide sequence is hard to predict, which makes peptide synthesis challenging. Review methods and reagents for avoiding aggregation in solid-phase peptide synthesis.
The ease of assembly of a given peptide sequence is hard to predict, which makes peptide synthesis challenging. Review methods and reagents for avoiding aggregation in solid-phase peptide synthesis.
The ease of assembly of a given peptide sequence is hard to predict, which makes peptide synthesis challenging. Review methods and reagents for avoiding aggregation in solid-phase peptide synthesis.
The ease of assembly of a given peptide sequence is hard to predict, which makes peptide synthesis challenging. Review methods and reagents for avoiding aggregation in solid-phase peptide synthesis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service