All Photos(1)
About This Item
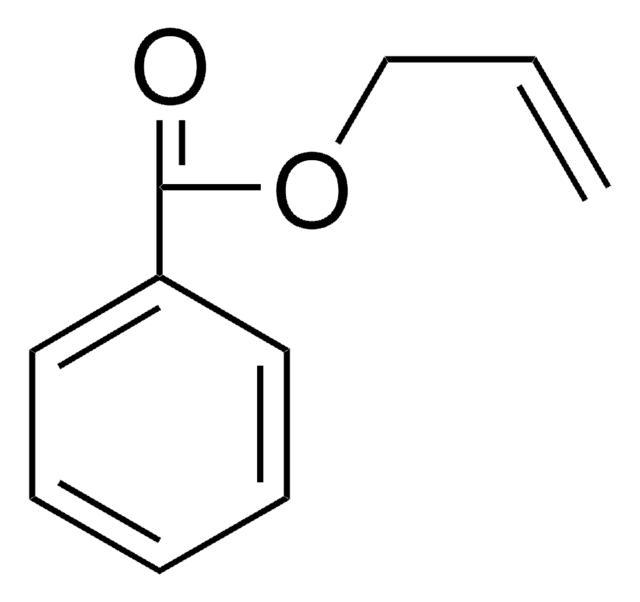
Linear Formula:
C6H5OCH2CH=CH2
CAS Number:
Molecular Weight:
134.18
Beilstein:
1905622
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.522 (lit.)
bp
192 °C (lit.)
density
0.978 g/mL at 25 °C (lit.)
SMILES string
C=CCOc1ccccc1
InChI
1S/C9H10O/c1-2-8-10-9-6-4-3-5-7-9/h2-7H,1,8H2
InChI key
POSICDHOUBKJKP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
145.4 °F - closed cup
Flash Point(C)
63 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anders Dahlén et al.
Organic letters, 5(22), 4085-4088 (2003-10-24)
[reaction: see text]. SmI2/H2O/amine provides selective cleavage of unsubstituted allyl ethers in good to excellent yields. This method is therefore useful in deprotection of alcohols and carbohydrates.
M Mahmoudian et al.
Applied microbiology and biotechnology, 37(1), 28-31 (1992-04-01)
Eighteen newly isolated ethene- and propene-utilizing bacteria were screened for the ability to produce phenyl glycidyl ether, a common precursor for the synthesis of beta blockers, from phenyl allyl ether. These organisms included Aerococcus, Alcaligenes, Micrococcus and Staphylococcus spp. and
Ralph Moser et al.
Organic letters, 12(1), 28-31 (2009-12-03)
Allylic phenyl ethers serve as electrophiles toward Pd(0) en route to a variety of allylic silanes. The reactions can be run at room temperature in water as the only medium using micellar catalysis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![Europium tris[3-(trifluoromethylhydroxymethylene)-(+)-camphorate]](/deepweb/assets/sigmaaldrich/product/structures/295/345/0e691b06-5215-407e-851b-d11f14f21a37/640/0e691b06-5215-407e-851b-d11f14f21a37.png)



