SCP0168
Histone Deacetylase Substrate
≥95% (HPLC), lyophilized
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C23H31N3O6
Molecular Weight:
445.51
UNSPSC Code:
12352204
NACRES:
NA.32
Recommended Products
product name
Histone Deacetylase Substrate,
Assay
≥95% (HPLC)
form
lyophilized
composition
Peptide Content, ≥85%
storage condition
protect from light
storage temp.
−20°C
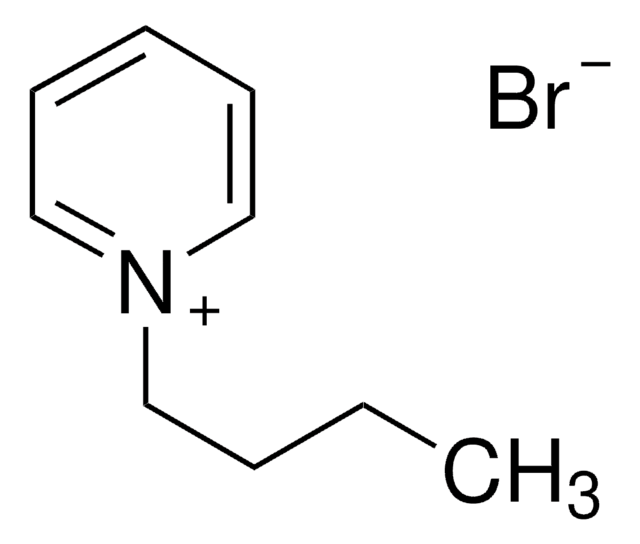
Amino Acid Sequence
BOC-Ac-Lys-AMC
Application
BOC-Ac-Lys-AMC (MAL) is used as a fluorogenic substrate for assaying both human zinc and NAD+-dependent histone deacetylases.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Birgit Heltweg et al.
Methods (San Diego, Calif.), 36(4), 332-337 (2005-08-10)
Histone deacetylases are important regulators of transcription and an emerging target for anticancer drugs. We present an overview over various assay formats that include radiolabelled histones, oligopeptides, and small molecules as substrates. The advantages and disadvantages of the various formats
Birgit Heltweg et al.
Analytical biochemistry, 319(1), 42-48 (2003-07-05)
Histone deacetylases (HDACs) are involved in the regulation of transcription and their inhibitors are a promising class of new anticancer drugs. We have previously reported Boc(Ac)Lys-AMC, also termed MAL, as a fluorescent substrate for HDACs. Now we present a modification
Neetinkumar D Reddy et al.
Chemico-biological interactions, 233, 81-94 (2015-04-01)
The potential of cinnamic acid as an anti-inflammatory and anti-cancer agent has been studied previously. In our investigation, novel bio-isosters of cinnamyl sulfonamide hydroxamate were synthesized, characterized and confirmed for their structure and evaluated for cytotoxicity. Three NCEs namely, NMJ-1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service