All Photos(1)
About This Item
Linear Formula:
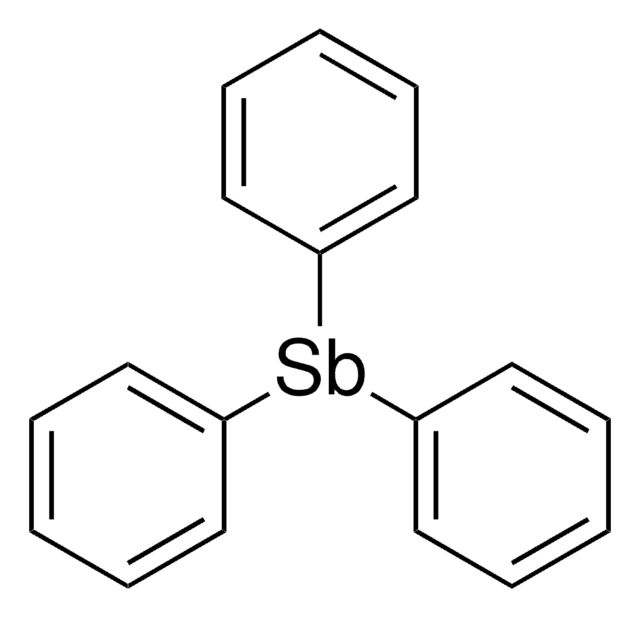
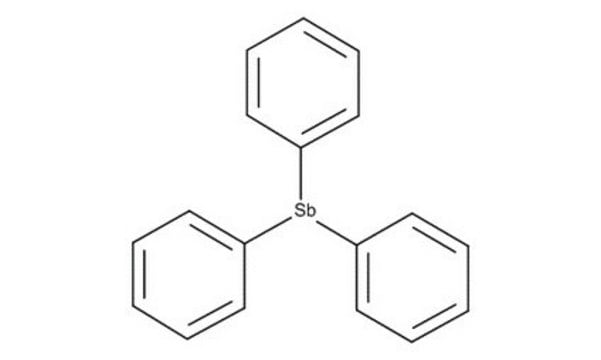
(C6H5)3As
CAS Number:
Molecular Weight:
306.23
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
solid
reaction suitability
reagent type: catalyst
mp
58-61 °C (lit.)
SMILES string
c1ccc(cc1)[As](c2ccccc2)c3ccccc3
InChI
1S/C18H15As/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15H
InChI key
BPLUKJNHPBNVQL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Triphenylarsine is an organoarsenic compound mainly used as a ligand and reagent in organic synthesis. It can also be used as a vapor deposition precursor to prepare nanomaterials.
Application
Triphenylarsine can be used:
- As a CVD precursor to prepare As-doped carbon nanotubes with enhanced activity and long-term durability for the oxygen reduction reaction.
- As an arsenic source to synthesize InAs nanocrystals.
- As a ligand to accelerate the reaction rate of the Stille coupling reaction.
- To prepare trichromophoric sensitizer for dye-sensitized solar cells.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Novel As-doped, As and N-codoped carbon nanotubes as highly active and durable electrocatalysts for O2 reduction in alkaline medium
Ziwu Liu, et al.
Journal of Power Sources, 306, 535-540 (2016)
Panchromatic Trichromophoric Sensitizer for Dye-Sensitized Solar Cells Using Antenna Effect
Julien Warnan, et al
Organic Letters, 13, 3944-3947 (2011)
Tertiary arsine ligands for the Stille coupling reaction
Akane Chishiro, et al.
Dalton Transactions, 51, 95-103 (2022)
Benzene-thermal route to InP and InAs nanocrystals using triphenylphosphine and triphenylarsine as pnicogen sources
Junli Wang and Qing Yang
Chemistry Letters (Jpn), 37, 306-307 (2008)
Ryoto Inaba et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 26(59), 13400-13407 (2020-07-15)
Efficient catalytic arsa-Wittig reactions have been developed by using 1-phenylarsolane as a catalyst. A wide array of aldehydes was converted to the corresponding olefins in high yields with moderate to excellent E stereoselectivity in the presence of a catalytic amount
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service