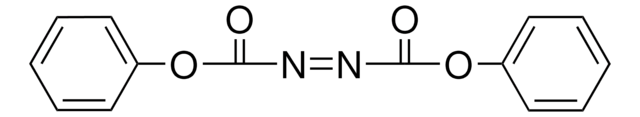680850
Di-(4-chlorobenzyl)azodicarboxylate
97%
Synonym(s):
Bis(4-chlorobenzyl)azodicarboxylate, DCAD
About This Item
Recommended Products
Assay
97%
form
solid
mp
108-112 °C
functional group
azo
chloro
SMILES string
O=C(/N=N\C(OCC1=CC=C(Cl)C=C1)=O)OCC2=CC=C(Cl)C=C2
InChI
1S/C16H12Cl2N2O4/c17-13-5-1-11(2-6-13)9-23-15(21)19-20-16(22)24-10-12-3-7-14(18)8-4-12/h1-8H,9-10H2/b20-19-
InChI key
UIFGGABIJBWRMG-VXPUYCOJSA-N
General description
Application
- Amino thioesters via guanidine-catalyzed biomimetic enantioselective decarboxylative Mannich and amination reactions of malonic acid half thioesters
- Hydroacylation reaction of aldehydes in Ionic liquid (IL) medium
- DCAD (di-p-chlorobenzyl azodicarboxylate) for Mitsunobu coupling reactions
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Designer surfactants enable diverse transformations like Suzuki-Miyaura, Olefin Metathesis, and 1,4-Addition to Enones in water by Prof. Bruce Lipshutz.
Designer surfactants enable diverse transformations like Suzuki-Miyaura, Olefin Metathesis, and 1,4-Addition to Enones in water by Prof. Bruce Lipshutz.
Designer surfactants enable diverse transformations like Suzuki-Miyaura, Olefin Metathesis, and 1,4-Addition to Enones in water by Prof. Bruce Lipshutz.
Designer surfactants enable diverse transformations like Suzuki-Miyaura, Olefin Metathesis, and 1,4-Addition to Enones in water by Prof. Bruce Lipshutz.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service