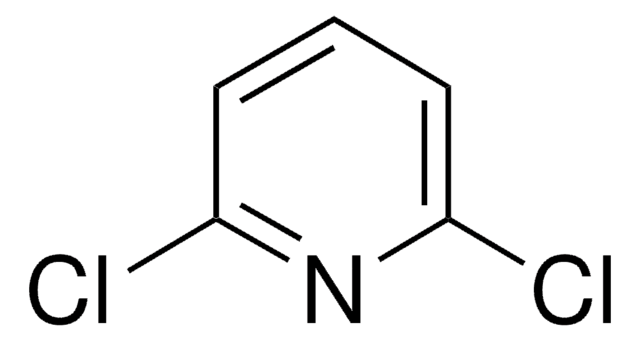
456543
2,6-Dichloropyridine-4-carboxylic acid
98%
Synonym(s):
2,6-Dichloroisonicotinic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H3Cl2NO2
CAS Number:
Molecular Weight:
192.00
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
209-212 °C (lit.)
functional group
carboxylic acid
chloro
SMILES string
OC(=O)c1cc(Cl)nc(Cl)c1
InChI
1S/C6H3Cl2NO2/c7-4-1-3(6(10)11)2-5(8)9-4/h1-2H,(H,10,11)
InChI key
SQSYNRCXIZHKAI-UHFFFAOYSA-N
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
European Journal of Organic Chemistry, 22, 4445-4449 (2003)
A Guo et al.
The Plant journal : for cell and molecular biology, 15(5), 647-656 (1998-10-21)
A clone encoding a putative soluble epoxide hydrolase (EH-1), an enzyme which converts epoxides to diols, was isolated by differential screening of a cDNA library prepared from tobacco mosaic virus (TMV)-infected tobacco leaves. To confirm that EH-1 encodes an epoxide
Uwe Conrath et al.
Trends in plant science, 7(5), 210-216 (2002-05-07)
Plants can acquire enhanced resistance to pathogens after treatment with necrotizing attackers, nonpathogenic root-colonizing pseudomonads, salicylic acid, beta-aminobutyric acid and many other natural or synthetic compounds. The induced resistance is often associated with an enhanced capacity to mobilize infection-induced cellular
The preparation of pyridine-4-carboxylic acid and of piperidine-4-carboxylic acid by catalytic reduction of 2, 6-dichloropyridine-4-carboxylic acid.
Wibaut JP.
Rec. Trav. Chim., 63(7), 141-146 (1944)
J M Manners et al.
Plant molecular biology, 38(6), 1071-1080 (1998-12-30)
The plant defensin PDF1.2 has previously been shown to accumulate systemically via a salicylic acid-independent pathway in leaves of Arabidopsis upon challenge by fungal pathogens. To further investigate the signalling and transcriptional processes underlying plant defensin induction, a DNA fragment
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service