141038
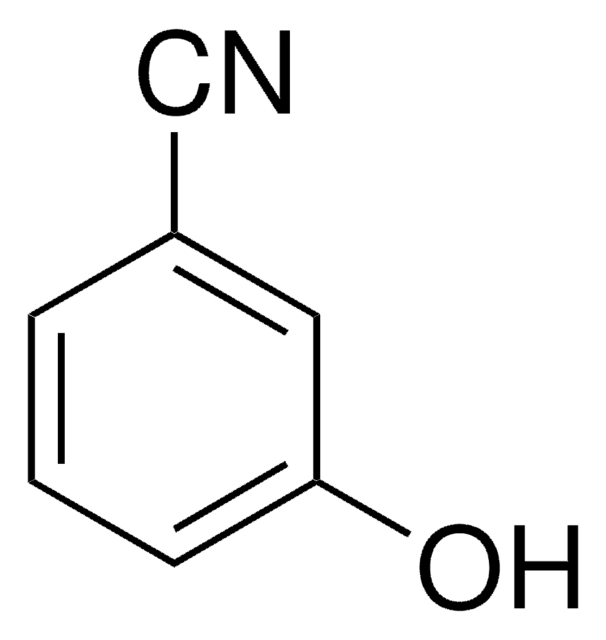
2-Hydroxybenzonitrile
99%
Synonym(s):
2-Cyanophenol, Salicylonitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NCC6H4OH
CAS Number:
Molecular Weight:
119.12
Beilstein:
1210029
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
powder
bp
149 °C/14 mmHg (lit.)
mp
92-95 °C (lit.)
functional group
nitrile
SMILES string
Oc1ccccc1C#N
InChI
1S/C7H5NO/c8-5-6-3-1-2-4-7(6)9/h1-4,9H
InChI key
CHZCERSEMVWNHL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Hydroxybenzonitrile may be used in the preparation of:
- methyl-(2-cyanophenoxy)acetate
- 3-amino-N-phenylbenzofuran-2-carboxamide
- 2-(2-oxo-2-phenylethoxy)benzonitrile
- 3-amino-2-cyanobenzo[b]furane
2-Hydroxybenzonitrile was used as starting reagent during the synthesis of:
- mono-alkoxyphenyloxazoline
- furanoside
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Sens. 1B
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enantioselective Cu-catalyzed 1, 4-addition of Me3Al to a 4, 4-disubstituted cyclohexa-2, 5-dienone.
Takemoto Y, et al.
Tetrahedron, 52(45), 14177-14188 (1996)
Reactivity of 1, 2-cyclic sulfite xylosides towards nucleophiles.
Batoux N, et al.
Tetrahedron, 65(43), 8858-8862 (2009)
Efficient New Synthesis of N-Arylbenzo [b] furo [3, 2-d] pyrimidin-4-amines and Their Benzo [b] thieno [3, 2-d] pyrimidin-4-amine Analogues via a Microwave-Assisted Dimroth Rearrangement.
Loidreau Y, et al.
Journal of Heterocyclic Chemistry, 50(5), 1187-1197 (2013)
meta-Selective arene C-H bond olefination of arylacetic acid using a nitrile-based directing group.
Bera M, et al.
Organic Letters, 16(21), 5760-5763 (2014)
A microwave-assisted multicomponent protocol for the synthesis of benzofuran-2-carboxamides.
Vincetti P, et al.
Tetrahedron Letters, 57(13), 1464-1467 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service