134228
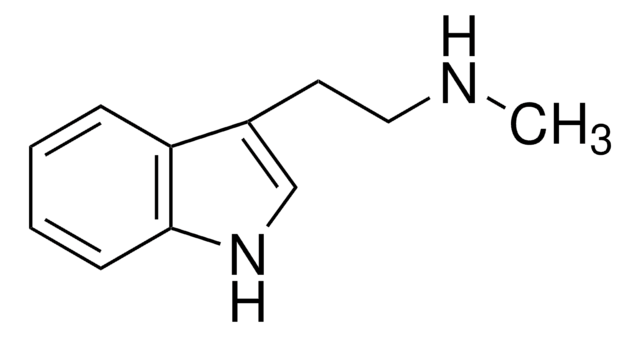
5-Methyltryptamine hydrochloride
98%
Synonym(s):
3-(2-Aminoethyl)-5-methylindole hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H14N2 · HCl
CAS Number:
Molecular Weight:
210.70
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
289-292 °C (dec.) (lit.)
solubility
H2O: soluble 50 mg/mL, clear, yellow
functional group
amine
SMILES string
Cl.Cc1ccc2[nH]cc(CCN)c2c1
InChI
1S/C11H14N2.ClH/c1-8-2-3-11-10(6-8)9(4-5-12)7-13-11;/h2-3,6-7,13H,4-5,12H2,1H3;1H
InChI key
RBHDFGBPJGEYCK-UHFFFAOYSA-N
Application
5-Methyltryptamine hydrochloride was used to study the mechanism of metabolism of 9-methyl 1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizine in rats. It was used as internal standard in the determination of urinary metabolites of 5-methoxy-N,N-diisopropyltryptamine in humans. It was used as reactant in:
- synthesis of kinesin spindle protein (KSP) inhibitors
- intramolecular furan Diels-Alder reactions
- Pictet-Spengler-like reactions
- reactant in synthesis of kinesin spindle protein (KSP) inhibitors
- reactant in intramolecular furan Diels-Alder reactions
- reactant in Pictet-Spengler-like reactions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
"Pictet-Spengler-like" Synthesis of Tetrahydro-beta-carbolines under Hydrolytic Conditions. Direct Use of Azalactones as Phenylacetaldehyde Equivalents.
James E. Audia et al.
The Journal of organic chemistry, 61(22), 7937-7939 (1996-11-01)
Ruan, X., et al.
Huaxue Xuebao, 66, 1731-1731 (2008)
Fokas, D., et al.
Tetrahedron Letters, 44, 5137-5137 (2003)
I Litosch et al.
The Journal of biological chemistry, 261(2), 638-643 (1986-01-15)
Incubation of blowfly salivary gland homogenates with 30 microM [gamma-32P]ATP resulted in a rapid, Mg2+-dependent, synthesis of [32P]polyphosphoinositides and [32P]phosphatidic acid. 5-Methyltryptamine, in the presence of 10 microM guanosine 5'-(3-O-thio)trisphosphate, reduced the net accumulation of 32P label into phosphatidylinositol-4,5-P2 and
J Sallés et al.
Psychopharmacology, 154(2), 115-125 (2001-04-21)
One of the major pathways for neurotransmitter signaling involves phosphoinositide-specific and G-protein-dependent phospholipase C-beta (PLC-beta), which stimulates the formation of inositol 1,4,5-trisphosphate and diacylglycerol. Serotonergic and muscarinic-cholinergic signals in the brain are largely mediated through the hydrolysis of phosphoinositides by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service