All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H6O2
CAS Number:
Molecular Weight:
134.13
Beilstein:
114632
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
bp
290 °C (lit.)
mp
71-74 °C (lit.)
SMILES string
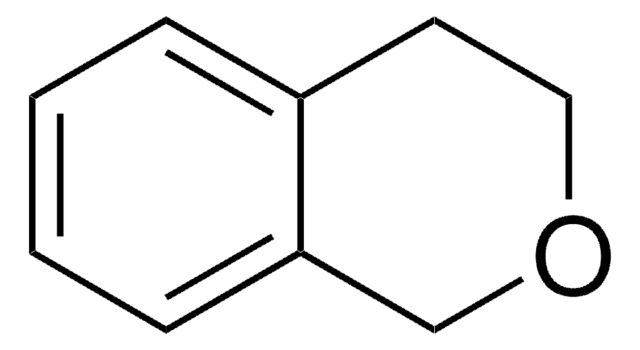
O=C1OCc2ccccc12
InChI
1S/C8H6O2/c9-8-7-4-2-1-3-6(7)5-10-8/h1-4H,5H2
InChI key
WNZQDUSMALZDQF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
305.6 °F - closed cup
Flash Point(C)
152 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chang-Yin Li et al.
Journal of pharmaceutical and biomedical analysis, 55(1), 146-160 (2011-02-01)
In this work, the metabolite profiles of Danggui Buxue Tang (DBT) in rat bile and plasma were qualitatively described, and the possible metabolic pathways of DBT were subsequently proposed. Emphasis was put on correlative analysis of metabolite profiling in different
Dario C Gerbino et al.
Organic letters, 14(9), 2338-2341 (2012-04-24)
The surprisingly facile conversion (isomerization) of 2-formyl-arylketones into 3-substituted phthalides, as observed for the marine natural product pestalone and its per-O-methylated derivative, was investigated using a series of simple 2-acylbenzaldehydes as substrates. The transformation generally proceeds smoothly in DMSO, either
Fangrui Zhong et al.
Journal of the American Chemical Society, 134(24), 10222-10227 (2012-05-25)
Phthalides were used for the first time in the allylic alkylation reactions with MBH carbonates for the creation of chiral 3,3-disubstituted phthalides. Highly enantioselective regiodivergent synthesis of γ-selective or β-selective allylic alkylation products was achieved by employing bifunctional chiral phosphines
Yun Wei et al.
Journal of chromatography. A, 1284, 53-58 (2013-03-15)
The phthalide compounds of Chuanxiong rhizoma including senkyunolide A, levistolide A, Z-ligustilide and 3-butylidenephthalide, have been reported as the biologically active compounds because of their therapeutic effects. In this work, online high-speed counter-current chromatography coupled with semi-preparative liquid chromatography instrument
Song-Hwa Chae et al.
Journal of agricultural and food chemistry, 59(15), 8193-8198 (2011-07-07)
The residual contact toxicity of three benzofuranoids (Z)-butylidenephthalide (1), (3S)-butylphthalide (2), and (Z)-ligustilide (3) identified in the rhizome of Cnidium officinale (Apiaceae) to B- and Q-biotype females of Bemisia tabaci was evaluated using a leaf-dip bioassay. Results were compared with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service