M6126
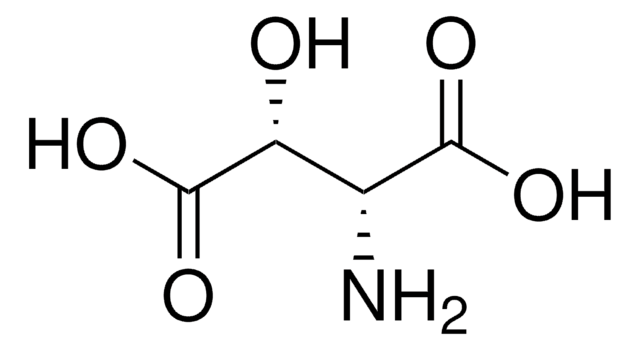
DL-threo-β-Methylaspartic acid
≥98% (TLC)
Synonym(s):
2-Amino-3-methylsuccinic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H9NO4
CAS Number:
Molecular Weight:
147.13
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product name
DL-threo-β-Methylaspartic acid,
Assay
≥98% (TLC)
form
powder
color
white
SMILES string
CC(C(N)C(O)=O)C(O)=O
InChI
1S/C5H9NO4/c1-2(4(7)8)3(6)5(9)10/h2-3H,6H2,1H3,(H,7,8)(H,9,10)
InChI key
LXRUAYBIUSUULX-UHFFFAOYSA-N
Biochem/physiol Actions
DL-threo-β-Methylaspartic acid is an amino acid derivative.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Silke Schabbert et al.
Bioorganic & medicinal chemistry, 10(10), 3331-3337 (2002-08-02)
We report the synthesis and biological activity of a series of side-chain-constrained RGD peptides containing the (2S,3R) or (2S,3S) beta-methyl aspartic acid within the RGD sequence. These compounds have been assayed for binding to the integrin receptors alpha(IIb)beta3 and alpha(v)beta3
Jorge Heredia-Moya et al.
Bioorganic & medicinal chemistry, 16(11), 5908-5913 (2008-05-13)
Beta-(S-Methyl)thioaspartic acid occurs as a posttranslational modification at position 88 in Escherichia coli ribosomal protein S12, a position that is a mutational hotspot resulting in both antibiotic-resistant and antibiotic-sensitive phenotypes. Critical to research designed to determine the biological function of
Miryam Asuncion et al.
The Journal of biological chemistry, 277(10), 8306-8311 (2001-12-19)
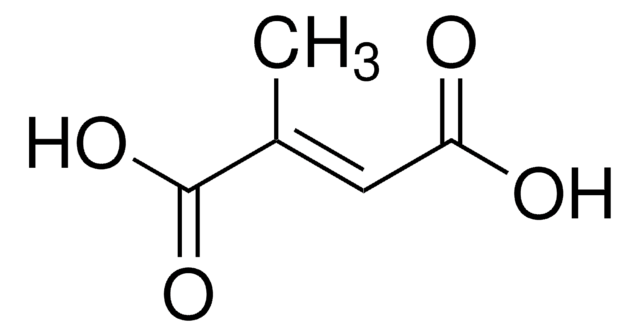
Methylaspartate ammonia-lyase (3-methylaspartase, MAL; EC ) catalyzes the reversible anti elimination of ammonia from L-threo-(2S,3S)-3-methylaspartic acid to give mesaconic acid. This reaction lies on the main catabolic pathway for glutamate in Clostridium tetanomorphum. MAL requires monovalent and divalent cation cofactors
G F Short et al.
Biochemistry, 39(30), 8768-8781 (2000-07-29)
Aspartates 25 and 125, the active site residues of HIV-1 protease, participate functionally in proteolysis by what is believed to be a general acid-general base mechanism. However, the structural role that these residues may play in the formation and maintenance
N P Botting et al.
Biochemistry, 31(5), 1509-1520 (1992-02-11)
The enzyme 3-methylaspartate ammonia-lyase (EC 4.3.1.2) catalyzes the exchange of the C-3 hydrogen of the substrate, (2S,3S)-3-methylaspartic acid, with solvent hydrogen. The mechanism of the exchange reaction was probed using (2S,3S)-3-methylaspartic acid and its C-3-deuteriated isotopomer. Incubations conducted in tritiated
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service