762016
3-Azido-1-propanamine
≥95%
Synonym(s):
3-Azidopropylamine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C3H8N4
CAS Number:
Molecular Weight:
100.12
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
form
liquid
reaction suitability
reaction type: click chemistry
refractive index
n20/D 1.471
density
1.020 g/mL at 25 °C
storage temp.
−20°C
SMILES string
NCCCN=[N+]=[N-]
InChI
1S/C3H8N4/c4-2-1-3-6-7-5/h1-4H2
InChI key
OYBOVXXFJYJYPC-UHFFFAOYSA-N
General description
3-Azido-1-propanamine can be used to functionalize:
- Bismethylolpropionic acid (bis-MPA) monomers with azide functional group to generate high-generation dendrimers.,
- Clickable zinc tetraphenylporphyrin scaffold with an azido group through click chemistry applicable in photodynamic therapy.
Application
Amine modified azide for click chemistry.
3-Azido-1-propanamine may be used in the synthesis of mannopyranoside dendrimers for studying multivalent carbohydrate-protein interactions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
140.0 °F
Flash Point(C)
60 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of mono-, di-and triporphyrin building blocks by click chemistry for photodynamic therapy application
Gazzali AM, et al.
Tetrahedron, 73(5), 532-541 (2017)
Xiaoqiang Chen et al.
Biomaterials, 122, 130-140 (2017-01-24)
The development of multifunctional reagents for simultaneous specific near-infrared (NIR) imaging and phototherapy of tumors is of great significance. This work describes the design of a cathepsin B-activated fluorescent probe (CyA-P-CyB) and its applications as an NIR imaging probe for
Anthony Angeli et al.
Chembiochem : a European journal of chemical biology, 18(11), 1036-1047 (2017-03-21)
Lectin A (LecA) from Pseudomonas aeruginosa is an established virulence factor. Glycoclusters that target LecA and are able to compete with human glycoconjugates present on epithelial cells are promising candidates to treat P. aeruginosa infection. A family of 32 glycodendrimers of generation 0
Mariano Ortega-Muñoz et al.
Nanoscale, 11(16), 7850-7856 (2019-04-10)
Activated carbon nanodots functionalized with acid anhydride groups (AA-CNDs) are prepared by one-pot water-free green thermolysis of citric acid. As a proof of concept of their capabilities as appealing and versatile platforms for accessing engineering nanoconstructs, the as-prepared AA-CNDs have
Dual display of proteins on the yeast cell surface simplifies quantification of binding interactions and enzymatic bioconjugation reactions.
Lim S, et al.
Biotechnology Journal, 12(5) (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service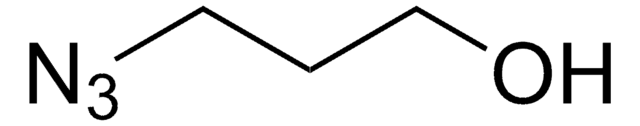


![2-[2-(2-Azidoethoxy)ethoxy]ethanol solution ~0.5 M in tert-butyl methyl ether](/deepweb/assets/sigmaaldrich/product/structures/374/007/eea7ca74-41e4-4aac-af71-c93c37ec0a5a/640/eea7ca74-41e4-4aac-af71-c93c37ec0a5a.png)