220639
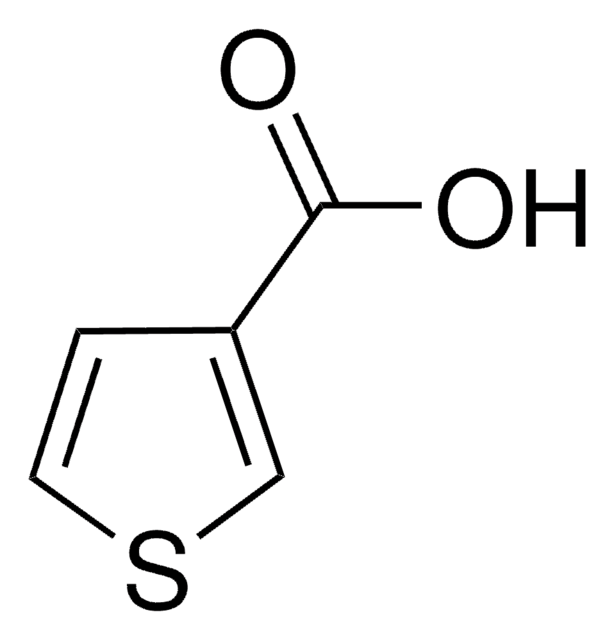
3-Thiopheneacetic acid
98%
Synonym(s):
3-Thienylacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6O2S
CAS Number:
Molecular Weight:
142.18
Beilstein:
113622
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
73-76 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)Cc1ccsc1
InChI
1S/C6H6O2S/c7-6(8)3-5-1-2-9-4-5/h1-2,4H,3H2,(H,7,8)
InChI key
RCNOGGGBSSVMAS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Thiopheneacetic acid acts as monocarboxylate ligand and forms oxo-carboxylate bridged digadolinium(III) complexes. Electrochemical oxidation of 3-thiopheneacetic acid in dry acetonitrile leads to the formation of a conducting polymeric film of poly (3-thiopheneacetic acid).
Application
3-Thiopheneacetic acid was used in:
- one-step, size control synthesis of gold nanoparticles
- in the preparation of gold nanoparticles capped with 3-thiopheneacetic acid (3-TAA) via borohydride reduction
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis, density functional theory, molecular dynamics and electrochemical studies of 3-thiopheneacetic acid-capped gold nanoparticles.
Sosibo NM, et al.
Journal of Molecular Structure, 1006(1), 494-501 (2011)
One-step, shape control synthesis of gold nanoparticles stabilized by 3-thiopheneacetic acid.
Huang H and Yang X.
Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 255(1), 11-17 (2005)
Laura Cañadillas-Delgado et al.
Dalton transactions (Cambridge, England : 2003), 39(31), 7286-7293 (2010-07-02)
Two new digadolinium(III) complexes with monocarboxylate ligands, [Gd2(pac)6(H2O)4] (1) and [Gd2(tpac)6(H2O)4] (2) (Hpac = pentanoic acid and Htpac = 3-thiopheneacetic acid), have been prepared and their structures determined by X-ray diffraction on single crystals. Their structures consist of neutral and
Electrochemistry of poly (3-thiopheneacetic acid) in aqueous solution: evidence for an intramolecular chemical reaction.
Bartlett PN and Dawson DH.
Journal of Materials Chemistry, 4(12), 1805-1810 (1994)
Muhammet Aydın et al.
Macromolecular bioscience, 19(8), e1900109-e1900109 (2019-06-22)
In this study, an impedimetric immunosensor based on polymer poly(thiophene)-graft-poly(methacrylamide) polymer (P(Thi-g-MAm)) modified indium tin oxide (ITO) electrode is developed for the detection of the Neuron Specific Enolase (NSE) cancer biomarker. First, the P(Thi-g-MAm) polymer is synthesized and coated on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service