Principles and Standard Conditions for Different Purification Techniques
Affinity chromatography (AC)
AC separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand attached to a chromatographic matrix. The technique is well-suited for a capture or intermediate step and can be used whenever a suitable ligand is available for the protein(s) of interest. AC offers high selectivity, hence high resolution, and usually high capacity [for the protein(s) of interest]. Affinity chromatography is frequently used as the first step (capture step) of a two-step purification protocol, followed by a second chromatographic step (polishing step) to remove remaining impurities.
The target protein(s) is specifically and reversibly bound by a complementary binding substance (ligand). The sample is applied under conditions that favor specific binding to the ligand. Unbound material is washed away, and the bound target protein is recovered by changing conditions to those favoring desorption. Desorption is performed specifically, using a competitive ligand, or nonspecifically, by changing the pH, ionic strength, or polarity. Samples are concentrated during binding, and protein is collected in purified and concentrated form. The key stages in an affinity chromatographic separation are shown in Figure A1.1. Affinity chromatography is also used to remove specific contaminants; for example, Benzamidine Sepharose 4 Fast Flow can remove serine proteases.

Ion exchange chromatography (IEX)
IEX separates proteins with differences in surface charge to give a very high resolution separation with high sample loading capacity. The separation is based on the reversible interaction between a charged protein and an oppositely charged chromatographic medium. Proteins bind as they are loaded onto a column. Conditions are then altered so that bound substances are eluted differentially. Elution is usually performed by increasing salt concentration or changing pH. Changes are made stepwise or with a continuous gradient. Most commonly, samples are eluted with salt (NaCl), using a gradient elution (Figure A1.2). Target proteins are concentrated during binding and collected in a purified, concentrated form.

Figure A1.2. Typical IEX gradient elution.
The net surface charge of proteins varies according to the surrounding pH. Typically, when above its isoelectric point (pI) a protein will bind to an anion exchanger; when below its pI a protein will bind to a cation exchanger. However, it should be noted that binding depends on charge and that surface charges may thus be sufficient for binding even on the other side of the pI. Typically IEX is used to bind the target molecule, but it can also be used to bind impurities if required. IEX can be repeated at different pH values to separate several proteins that have distinctly different charge properties, as shown in Figure A1.3.

Figure A1.3. Effect of pH on protein elution patterns.
Method development (in priority order)
- Select optimal ion exchanger using small columns as in the HiTrap IEX Selection Kit to save time and sample.
- Scout for optimal pH to maximize capacity and resolution. Begin 0.5 to 1 pH unit away from the isoelectric point of the target protein if known.
- Select the steepest gradient to give acceptable resolution at the selected pH.
- Select the highest flow rate that maintains resolution and minimizes separation time. Check recommended flow rates for the specific medium.
To reduce separation times and buffer consumption, transfer to a step elution after method optimization as shown in Figure A1.4. It is often possible to increase sample loading when using step elution.
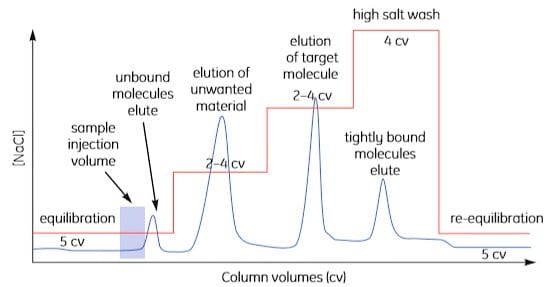
Figure A1.4. Step elution.
Hydrophobic interaction chromatography (HIC)
HIC separates proteins with differences in hydrophobicity. The technique is well-suited for the capture or intermediate steps in a purification protocol. Separation is based on the reversible interaction between a protein and the hydrophobic surface of a chromatographic medium. This interaction is enhanced by high ionic strength buffer, which makes HIC an excellent “next step” after precipitation with ammonium sulfate or elution in high salt during IEX. Samples in high ionic strength solution (e.g., 1.5 M ammonium sulfate) bind as they are loaded onto a column. Conditions are then altered so that the bound substances are eluted differentially.
Elution is usually performed by decreases in salt concentration (Figure A1.5). Changes are made stepwise or with a continuous decreasing salt gradient. Most commonly, samples are eluted with a decreasing gradient of ammonium sulfate. Target proteins are concentrated during binding and collected in a purified and concentrated form. Other elution procedures include reducing eluent polarity (ethylene glycol gradient up to 50%), adding chaotropic species (urea, guanidine hydrochloride) or detergents, changing pH or temperature.

Figure A1.5. Typical HIC gradient elution.
Method development (in priority order)
- The hydrophobic behavior of a protein is difficult to predict, and binding conditions must be studied carefully. Use HiTrap HIC Selection Kit or RESOURCE HIC Test Kit to select the medium that gives optimal binding and elution over the required range of salt concentration. For proteins with unknown hydrophobic properties begin with 0–100% elution buffer (0% elution buffer, e.g., 1 M ammonium sulfate). Knowledge about the solubility of the protein in the binding buffer is important because high concentrations of, for example, ammonium sulfate may precipitate proteins.
- Select the gradient that gives acceptable resolution.
- Select the highest flow rate that maintains resolution and minimizes separation time. Check recommended flow rates for the specific medium.
- If samples adsorb strongly to a medium then conditions that cause conformational changes, such as pH, temperature, chaotropic ions, or organic solvents can be altered. Conformational changes caused by these agents are specific to each protein. Use screening procedures to investigate the effects of these agents. Alternatively, change to a less hydrophobic medium.
To reduce separation times and buffer consumption, transfer to a step elution after method optimization, as shown in Figure A1.6. It is often possible to increase sample loading when using step elution.
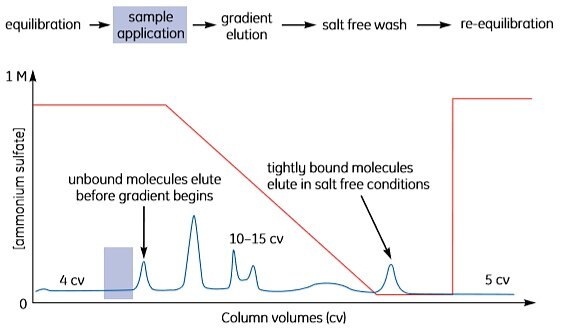
Figure A1.6. Step elution.
Gel filtration (GF) chromatography
GF separates proteins with differences in molecular size. The technique is well-suited for the final polishing steps in purification when sample volumes have been reduced (sample volume significantly influences speed and resolution in gel filtration). Samples are eluted isocratically (single buffer, no gradient, Figure A1.7). Buffer conditions are varied to suit the sample type or the requirements for further purification, analysis, or storage, because buffer composition usually does not have major effects on resolution. Proteins are collected in purified form in the chosen buffer.

Figure A1.7. Typical GF elution.
Reversed phase chromatography (RPC)
RPC separates proteins and peptides with differing hydrophobicity based on their reversible interaction with the hydrophobic surface of a chromatographic medium. Samples bind as they are loaded onto a column. Conditions are then altered so that the bound substances are eluted differentially. Due to the nature of the reversed phase matrices, the binding is usually very strong. Binding may be modulated by the use of organic solvents and other additives (ion pairing agents). Elution is usually performed by increases in organic solvent concentration, most commonly acetonitrile. Samples, which are concentrated during the binding and separation process, are collected in a purified, concentrated form. The key stages in a separation are shown in Figure A1.8.

Figure A1.8. Typical RPC gradient elution.
RPC is often used in the final polishing of oligonucleotides and peptides and is well-suited for analytical separations, such as peptide mapping.
RPC is not recommended for protein purification if recovery of activity and return to a correct tertiary structure are required, because many proteins are denatured in the presence of organic solvents.
Method development
- Select medium from screening results.
- Select optimal gradient to give acceptable resolution. For unknown samples begin with 0–100% elution buffer.
- Select highest flow rate that maintains resolution and minimizes separation time.
- For large-scale purification transfer to a step elution.
- Samples that adsorb strongly to a medium are more easily eluted by changing to a less hydrophobic medium.
To continue reading please sign in or create an account.
Don't Have An Account?