Using Human iPS Cell-derived Colon Organoids for Cytotoxicity Screening of Drug Compounds
Kevin Su, Nick Asbrock, Vi Chu, Ph.D., Stefanie Hoffmann, M.S., Philip Hewitt, Ph.D.
Inconclusive or adverse screening results of the effects of drugs and their metabolites on relevant human tissues have negatively impacted the ability to introduce new drug candidates. Additionaly, absorption of orally delivered drugs is a crucial criterion for screening new compounds at the early stage of drug discovery.1 Monolayers of Caco‐2 cells, a transformed cell line derived from human colorectal carcinoma, have been widely used by pharmaceutical companies as a standard intestinal barrier model for the prediction of intestinal permeability for drug candidates.2 Despite their convenience and accessibility, Caco-2 cells have a single homogeneous phenotype, and therefore fail to recapitulate lineage development into the diverse cell phenotypes that constitute the physiology of the native intestinal epithelium, leading to inconclusive experimental results. Stem cell- and tissue- derived organoids have more complex 3D tissue-like architecture and structure.3 Human epithelial colon organoids are thought to be considered more physiologically relevant for drug screening applications.4 We have generated a highly-qualified human iPSC-derived colonic organoid system consisting of assay-ready cryopreserved human colon organoids and specialized serum-free expansion media that was used to test the cytotoxic effects of numerous drug compounds.
Organoid Drug Screening Protocol
- Harvest four Matrigel® matrix domes that contain human colonic organoids (SCC300).
- Split organoids using the 3dGRO™ Organoid Dissociation Reagent (SCM300) following recommended instructions.
- Seed 10 µL Matrigel® matrix of a matrix-iPSC derived colon organoid solution into 96-well plates (white/clear bottom).
- Incubate matrix domes for 10 minutes at 37 °C, 5% CO2.
- Add 100 µL per well of the 3dGRO™ Human Colon Organoid Expansion Media (SCM304), 10 µM ROCKi (SCM075).
- Incubate organoids for 1 day at 37 °C, 5% CO2.
- On the next day, aspirate old media and dispense 100 µL into each well of fresh 3dGRO™ Colon Organoid Expansion Media (SCM304), 10 µM ROCKi (SCM075).
- Incubate organoids at 37 °C, 5% CO2 for 3 days.
- Prepare selected test compounds in their desired concentrations.
- Aspirate spent media.
- Add 100 µL/well of treatment solution (compound + media).
- Incubate at 37 °C & 5% CO2 for 72 hours.
- Bring the plate to room temperature for 30 minutes.
- Dispense 100 µL/well CellTiter-Glo® 3D Cell Viability Reagent.
- Cover the plate with aluminium foil and shake for 5 minutes at room temperature.
- Incubate plate for 25 minutes at room temperature.
- Measure luminescence on a plate reader.
- Calculate IC50 values.
Protocol Tips
- Thoroughly mix the matrix/organoid solution to achieve similar organoid numbers per well before cell seeding.
- Check viability of organoids via microscopy before adding drug compounds.
- Check number of organoids via microscopy to normalize data on cell count after treatment

Figure 1.Morphology and growth of untreated human colon organoids. Human colonic organoids are expanded in 3D Matrigel® matrix domes for a 10 day period and increase in overall mean length and area.
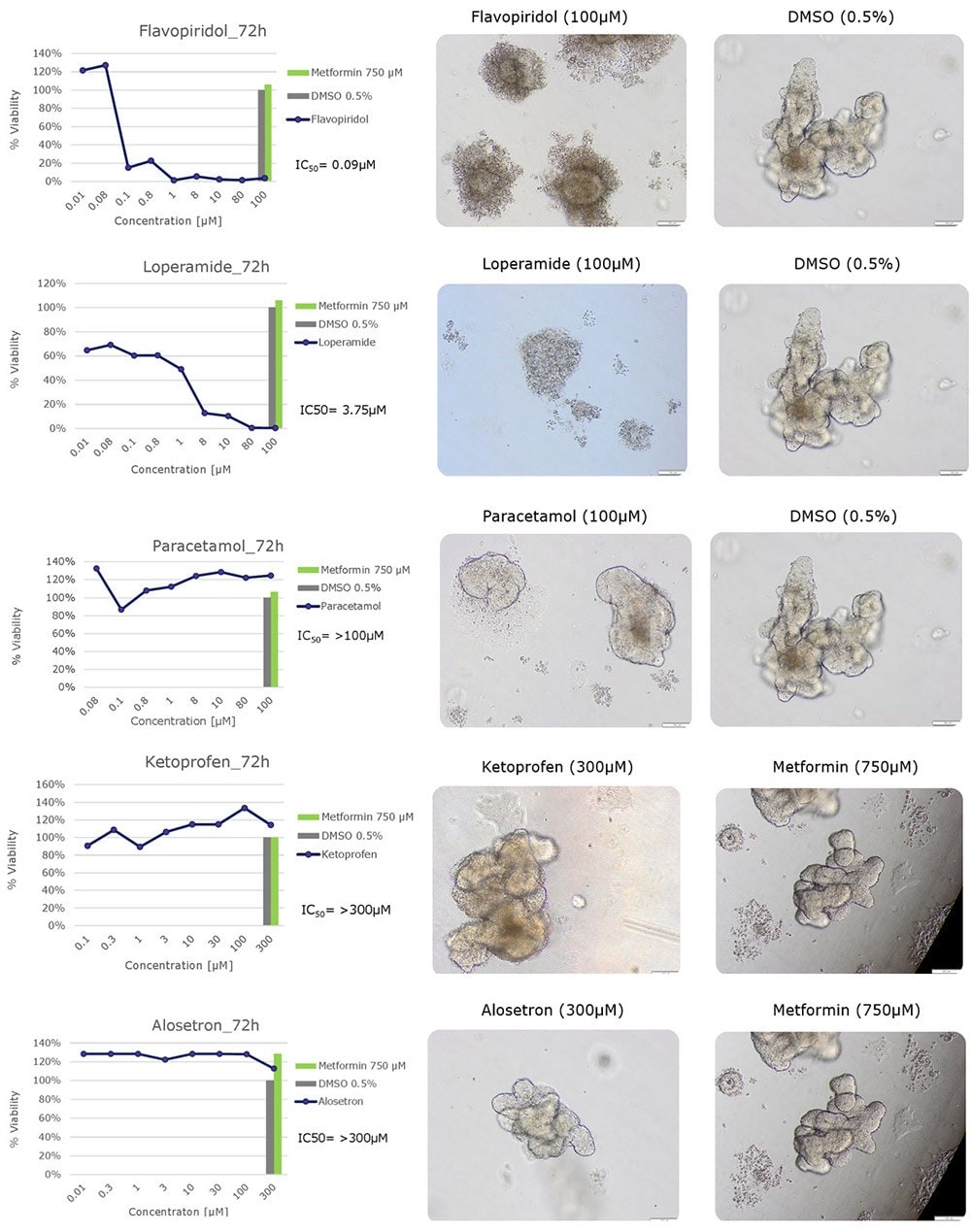
Figure 2.Cytotoxicity testing of drug compounds using human colon organoids. Cytotoxic effects of five compounds (flavopiridol, loperamide, paracetamol, ketoprofen and alosetron) on human colon organoids using the CellTiter-Glo® 3D Cell Viability Reagent.
Conclusion
Here we demonstrate the use of highly-characterized human iPS cell-derived colon organoids as an alternative cell model for traditional Caco-2 cell drug toxicity assays for drug screening applications. Cell viability of five compounds were tested: two compounds (flavopiridol and loperamide) had negative effects on cell viability, three compounds (paracetamol, ketoprofen, alosetron) had no effects on cell viability. Using human colonic organoids as an intestinal tissue model could decrease the time and cost of drug development and provide new insights into drug absorption and drug discovery.
References
To continue reading please sign in or create an account.
Don't Have An Account?