761605
BrettPhos Pd G3
98%
Synonym(s):
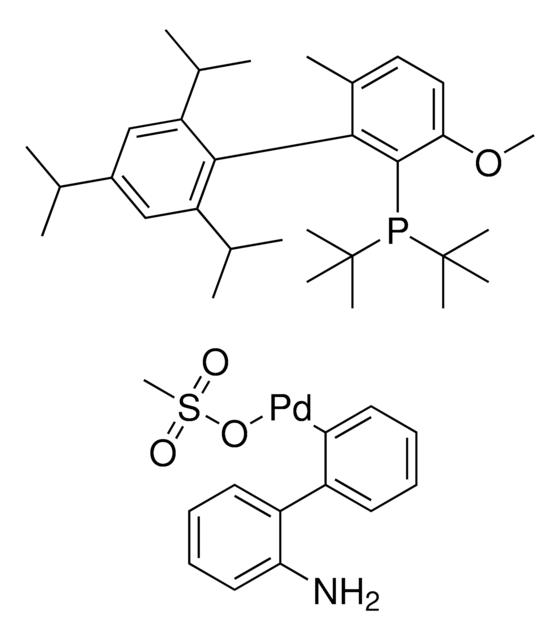
[(2-Di-cyclohexylphosphino-3,6-dimethoxy-2′,4′,6′- triisopropyl-1,1′-biphenyl)-2-(2′-amino-1,1′ -biphenyl)]palladium(II) methanesulfonate methanesulfonate
About This Item
Recommended Products
assay
98%
form
solid
feature
generation 3
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: catalyst
core: palladium
reaction type: Cross Couplings
mp
150-193 °C (decomposition)
functional group
amine
SMILES string
CS(=O)(O[Pd]c1c(c2c(N)cccc2)cccc1)=O.COc3c(P(C4CCCCC4)C5CCCCC5)c(c6c(C(C)C)cc(C(C)C)cc6C(C)C)c(OC)cc3
InChI
1S/C35H53O2P.C12H10N.CH4O3S.Pd/c1-23(2)26-21-29(24(3)4)33(30(22-26)25(5)6)34-31(36-7)19-20-32(37-8)35(34)38(27-15-11-9-12-16-27)28-17-13-10-14-18-28;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h19-25,27-28H,9-18H2,1-8H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1
InChI key
PQYBJDCHLVJYSD-UHFFFAOYSA-M
General description
Application
It may be used in the synthesis of following compounds:
- [Pd(cinnamyl)(BrettPhos)]OTf by reacting with [(cinnamyl)PdCl]2 and AgOTf.
- [Pd(crotyl)(BrettPhos)]OTf by reacting with [(crotyl)PdCl]2 and AgOTf.
- [Pd(allyl)(BrettPhos)]OTf by reacting with [(allyl)PdCl]2 and AgOTf.
- Pd(allyl)(BrettPhos)Clby reacting with [(allyl)PdCl]2.
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Preformed catalysts in the kit are stable but become air-sensitive when activated; Schlenk technique aids scale-up.
All contents in the foil bag are weighed, plated, packed, and sealed in a glove box under nitrogen.
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
Related Content
The Buchwald group has developed a series of highly active and versatile palladium precatalysts and biarylphosphine ligands used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. The ligands are electron-rich, and highly tunable to provide catalyst systems with a diverse scope, high stability and reactivity. Furthermore, the new series of precatalysts are air-, moisture and thermally-stable and display good solubility in common organic solvents. The use of precatalysts ensures the efficient generation of the active catalytic species and allows one to accurately adjust the ligand:palladium ratio. The ligands, precatalysts and methodology developed in the Buchwald group are user friendly and have rendered previously difficult cross couplings reactions, much easier to achieve.
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service